Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and method of administration** Treatment should be initiated under the supervision of a physician experienced in the management of multiple sclerosis (MS). Posology The recommended dose is 0.92 mg ozanimod once daily. The initial dose escalation regimen of ozanimod from Day 1 to Day 7 is required and shown below in Table 1. Following the 7-day dose escalation, the once daily dose is 0.92 mg, starting on Day 8. 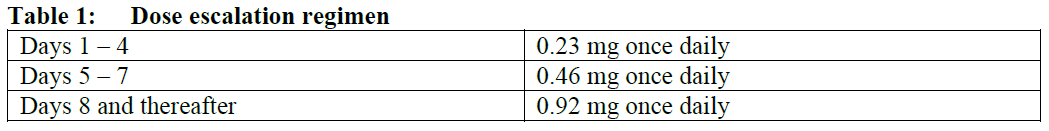 _Re-initiation of therapy following treatment interruption_ The same dose escalation regimen described in Table 1 is recommended when treatment is interrupted for: - 1 day or more during the first 14 days of treatment. - more than 7 consecutive days between Day 15 and Day 28 of treatment. - more than 14 consecutive days after Day 28 of treatment. If the treatment interruption is of shorter duration than the above, the treatment should be continued with the next dose as planned. _Special populations_ _Adults over 55 years old and elderly population_ There are limited data available on RRMS patients > 55 years of. No dose adjustment is needed in patients over 55 years of age. Caution should be used in MS patients over 55, given the limited data available and potential for an increased risk of adverse reactions in this population, especially with long-term treatment (see section 5.1 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ No dose adjustment is necessary for patients with renal impairment. _Hepatic impairment_ Patients with mild or moderate chronic hepatic impairment (Child-Pugh class A or B) are recommended to complete the 7-day dose escalation regimen, and then take 0.92 mg once every other day (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Ozanimod was not evaluated in patients with severe hepatic impairment. Therefore, patients with severe hepatic impairment (Child-Pugh class C) must not be treated with ozanimod (see sections 4.3 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ The safety and efficacy of Zeposia in children and adolescents aged below 18 years have not yet been established. No data are available. Method of administration Oral use. The capsules can be taken with or without food.
ORAL
Medical Information
**4.1 Therapeutic indications** Multiple sclerosis Zeposia is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis (RRMS) with active disease as defined by clinical or imaging features, to decrease the frequency of clinical exacerbations. (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. - Immunodeficient state (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Patients who in the last 6 months experienced myocardial infarction (MI), unstable angina, stroke, transient ischaemic attack (TIA), decompensated heart failure requiring hospitalisation or New York Heart Association (NYHA) Class III/IV heart failure. - Patients with history or presence of second-degree atrioventricular (AV) block Type II or third-degree AV block or sick sinus syndrome unless the patient has a functioning pacemaker. - Severe active infections, active chronic infections such as hepatitis and tuberculosis (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Active malignancies. - Severe hepatic impairment (Child-Pugh class C). - During pregnancy and in women of childbearing potential not using effective contraception (see sections 4.4 and 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Patients who are taking a monoamine oxidase (MAO) inhibitor.
L04AA38
xl 04 aa 38
Manufacturer Information
BRISTOL-MYERS SQUIBB (SINGAPORE) PTE. LTD.
Celgene International Sàrl
Active Ingredients
Documents
Package Inserts
Zeposia capsules PI.pdf
Approved: May 17, 2023
