Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2 DOSAGE AND ADMINISTRATION** **2.1 Recommended Dosage for Multiple Myeloma** In Combination with Bortezomib and Dexamethasone (SVd) The recommended dosage of XPOVIO is 100 mg taken orally once weekly on Day 1 of each week until disease progression or unacceptable toxicity in combination with: - Bortezomib 1.3 mg/m2 administered subcutaneously once weekly on Day 1 of each week for 4 weeks followed by 1 week off. - Dexamethasone 20 mg taken orally twice weekly on Days 1 and 2 of each week. Refer to _Clinical Studies (14.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ and the prescribing information of bortezomib and dexamethasone for additional dosing information. In Combination with Dexamethasone (Sd) The recommended dosage of XPOVIO is 80 mg taken orally on Days 1 and 3 of each week until disease progression or unacceptable toxicity in combination with dexamethasone 20 mg taken orally with each dose of XPOVIO on Days 1 and 3 of each week. For additional information regarding the administration of dexamethasone, refer to its prescribing information. **2.2 Recommended Dosage for Diffuse Large B‐Cell Lymphoma** The recommended dosage of XPOVIO is 60 mg taken orally on Days 1 and 3 of each week until disease progression or unacceptable toxicity. **2.3 Recommended Monitoring for Safety** Monitor complete blood count (CBC) with differential, standard blood chemistries, body weight, nutritional status, and volume status at baseline and during treatment as clinically indicated. Monitor more frequently during the first three months of treatment _\[see Warning and Precautions (5.1, 5.2, 5.3, and 5.4)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Assess the need for dosage modifications of XPOVIO for adverse reactions _\[see Dosage and Administration (2.5)\]_. **2.4 Recommended Concomitant Treatments** Advise patients to maintain adequate fluid and caloric intake throughout treatment. Consider intravenous hydration for patients at risk of dehydration _\[see Warnings and Precautions (5.3, 5.4)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Provide prophylactic antiemetics. Administer a 5‐HT3 receptor antagonist and other anti‐nausea agents prior to and during treatment with XPOVIO _\[see Warnings and Precautions (5.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.5 Dosage Modification for Adverse Reactions** Recommended XPOVIO dosage reduction steps are presented in Table 1. 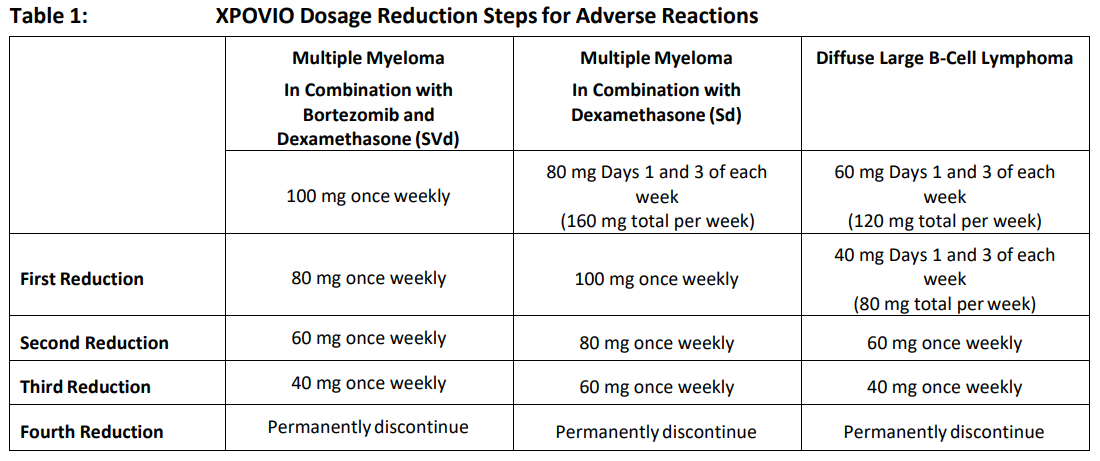 Recommended dosage modifications for hematologic adverse reactions in patients with multiple myeloma and DLBCL are presented in Table 2 and Table 3, respectively. Recommended dosage modifications for non‐hematologic adverse reactions are presented in Table 4. 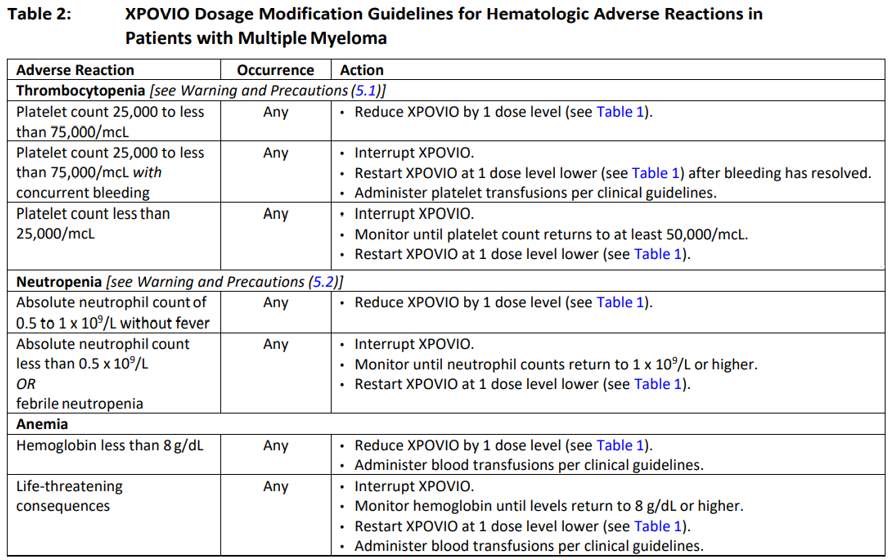 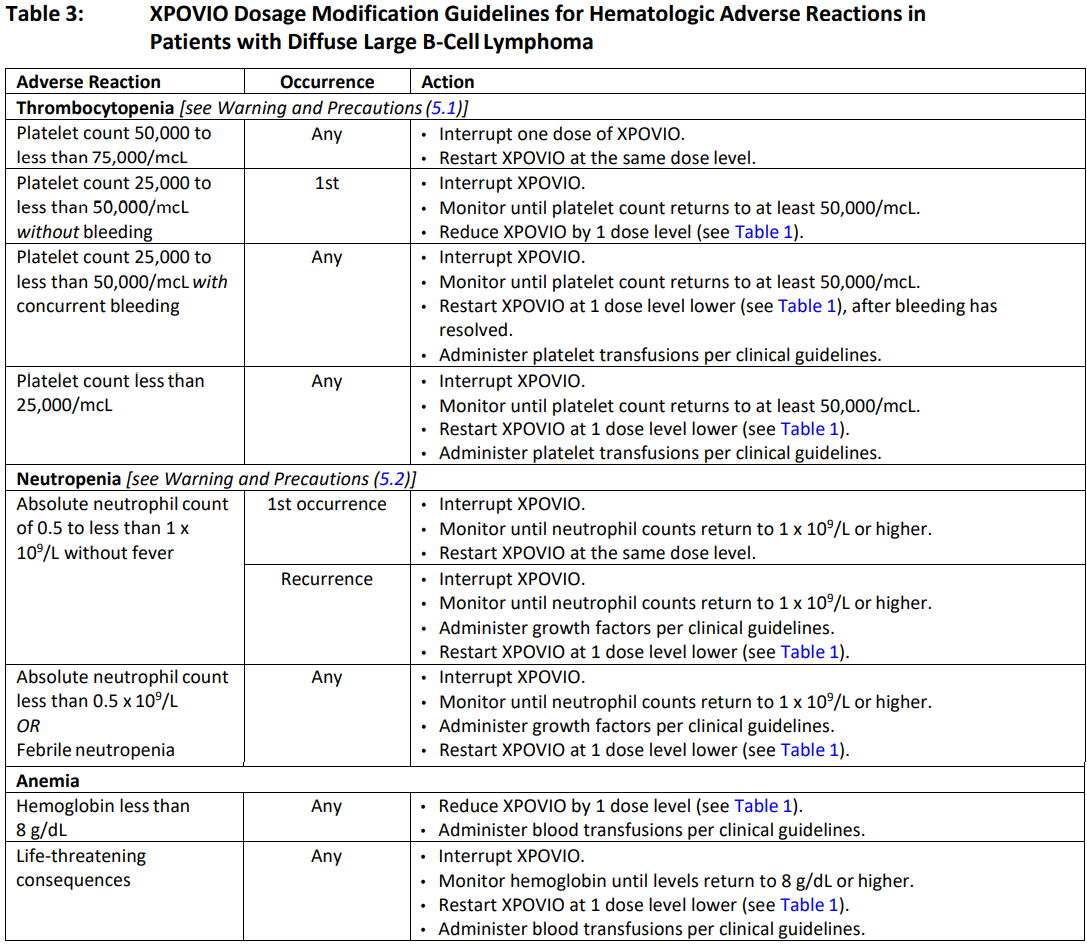 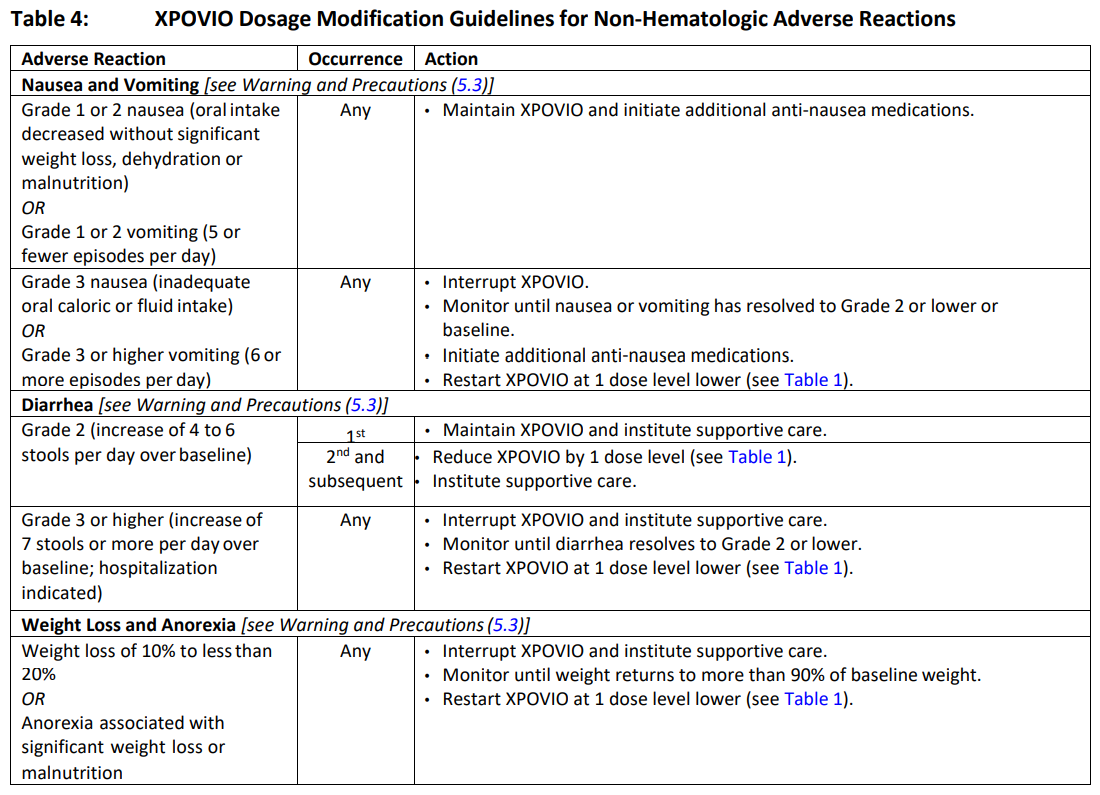 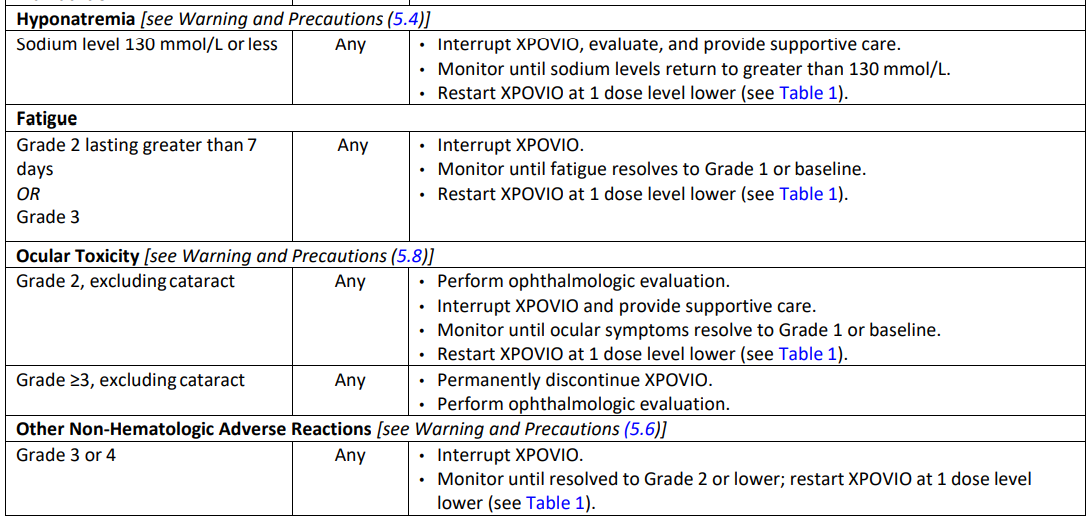 **2.6 Administration** Each XPOVIO dose should be taken at approximately the same time of day and each tablet should be swallowed whole with water. Do not break, chew, crush, or divide the tablets. If a dose of XPOVIO is missed or delayed, instruct patients to take their next dose at the next regularly scheduled time. If a patient vomits a dose of XPOVIO, the patient should not repeat the dose and the patient should take the next dose on the next regularly scheduled day.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** **1.1 Multiple Myeloma** - XPOVIO in combination with bortezomib and dexamethasone is indicated for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. - XPOVIO in combination with dexamethasone is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti‐CD38 monoclonal antibody. **1.2 Diffuse Large B‐Cell Lymphoma** XPOVIO is indicated for the treatment of adult patients with relapsed or refractory diffuse large B‐cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least 2 lines of systemic therapy who are not eligible for haematopoietic cell transplant \[see Clinical Studies (14.2) – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
**4 CONTRAINDICATIONS** None.
L01XX66
selinexor
Manufacturer Information
ANTENGENE (SINGAPORE) PTE. LTD.
Catalent CTS, LLC
Pharma Packaging Solutions, LLC (Primary and Secondary Packager)
Active Ingredients
Documents
Package Inserts
Xpovio PI.pdf
Approved: March 1, 2022
