Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** HALAVEN® should be administered only under the supervision of a qualified physician experienced in the appropriate use of cytotoxic drugs. The recommended dose of HALAVEN® as the ready to use solution is 1.4 mg/m2 which should be administered intravenously over 2–5 minutes on Days 1 and 8 of every 21-day cycle _Dose delays during therapy_ Delay the administration of HALAVEN® on Day 1 or Day 8 for any of the following: - Absolute neutrophil count (ANC) < 1 x 109/l - Platelets < 75 x 109/l - Grade 3 or 4 non-hematological toxicities. _Dose reduction during therapy_ Patients should be clinically evaluated during treatment by physical examination and laboratory testing including complete blood counts (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If Grade 3 or 4 toxicities are present, then treatment should be delayed to allow recovery. Patients should only be retreated when the ANC is ≥ 1 x 109/l and platelets are ≥ 75 x 109/l and all other toxicity from a previous cycle has recovered to Grade 2 or less. Dose reduction recommendations for retreatment are shown in the following table. If toxicities reoccur, an additional dose reduction should be made as shown. **Dose reduction recommendations** 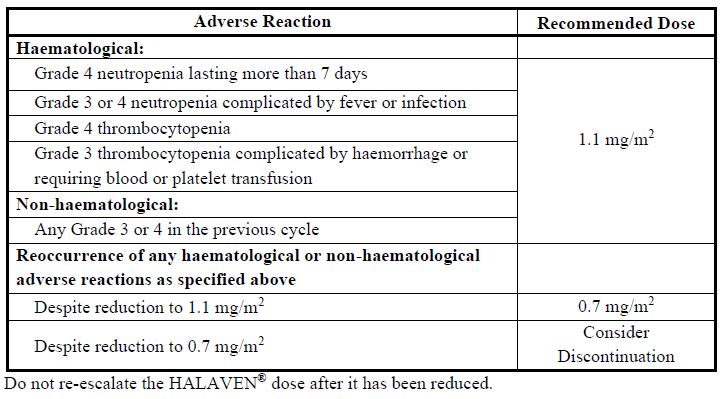 Patients with hepatic impairment _Impaired liver function due to metastases_ The recommended dose of HALAVEN® in patients with mild hepatic impairment (Child-Pugh A) is 1.1 mg/m2 administered intravenously over 2 to 5 minutes on Days 1 and 8 of a 21-day cycle. The recommended dose of HALAVEN® in patients with moderate hepatic impairment (Child-Pugh B) is 0.7 mg/m2 administered intravenously over 2 to 5 minutes on Days 1 and 8 of a 21-day cycle. Severe hepatic impairment (Child-Pugh C) has not been studied but it is expected that a more marked dose reduction is needed if HALAVEN® is used in these patients. _Impaired liver function due to cirrhosis_ This patient group has not been studied. The doses above may be used in mild and moderate impairment but close monitoring is advised as the doses may need readjustment. Paediatric patients The safety and effectiveness of HALAVEN® in paediatric patients below the age of 18 years have not been established. Elderly patients Clinical studies did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. There was no evidence to suggest that the safety profile of HALAVEN® is different in elderly patients. No specific dose adjustments are recommended based on the age of the patient (see section 4.8 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with renal impairment Some patients with moderately or severely impaired renal function (creatinine clearance <50 ml/min) may have increased eribulin exposure and may need a reduction of the dose. For all patients with renal impairment, caution and close safety monitoring is advised. (See Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Method of administration HALAVEN® is for intravenous use. The dose may be diluted in up to 100 ml of sodium chloride 9 mg/ml (0.9%) solution for injection. It should not be diluted in glucose 5% infusion solution. For instructions on the dilution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Good peripheral venous access, or a patent central line, should be ensured prior to administration. There is no evidence that eribulin mesilate is a vesicant or an irritant. In the event of extravasation, treatment should be symptomatic. For information relevant to the handling of cytotoxic medicinal products see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS BOLUS
Medical Information
**4.1 Therapeutic indications** HALAVEN® is indicated for the treatment of patients with locally advanced or metastatic breast cancer who have progressed after at least two chemotherapeutic regimens for advanced disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting. HALAVEN® is indicated for the treatment of adult patients with unresectable liposarcoma who have received prior anthracycline containing therapy (unless unsuitable) for advanced or metastatic disease (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ - Breast feeding
L01XX41
eribulin
Manufacturer Information
EISAI (SINGAPORE) PTE. LTD.
NERPHARMA S.R.L.
BSP Pharmaceuticals S.p.A (DP & Primary packager)
Active Ingredients
Documents
Package Inserts
HALAVEN _PI.pdf
Approved: June 19, 2023
