Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2. Posology and method of administration** _Posology_ _Adults_ **Monotherapy and combination with other oral antidiabetic agents** The usual starting dose is 500 mg or 850 mg metformin hydrochloride 2 or 3 times daily given during or after meals. After 10 to 15 days the dose should be adjusted on the basis of blood glucose measurements. A slow increase of dose may improve gastrointestinal tolerability. In patients receiving a high metformin dose (2 to 3 grams per day), it is possible to replace two Glucophage 500 mg, film-coated tablets with one Glucophage 1000 mg, film-coated tablet. The maximum recommended dose of metformin hydrochloride is 3 g daily, taken as 3 divided doses. If transfer from another oral antidiabetic agent is intended: discontinue the other agent and initiate metformin at the dose indicated above. **Combination with insulin** Metformin and insulin may be used in combination therapy to achieve better blood glucose control. Metformin hydrochloride is given at the usual starting dose of 500 mg or 850 mg 2 or 3 times daily, while insulin dosage is adjusted on the basis of blood glucose measurements. _Elderly_ Due to the potential for decreased renal function in elderly subjects, the metformin dosage should be adjusted based on renal function. Regular assessment of renal function is necessary (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Patients with renal impairment_ A GFR should be assessed before initiation of treatment with metformin containing products and at least annually thereafter. In patients at an increased risk of further progression of renal impairment and in the elderly, renal function should be assessed more frequently, e.g. every 3–6 months. 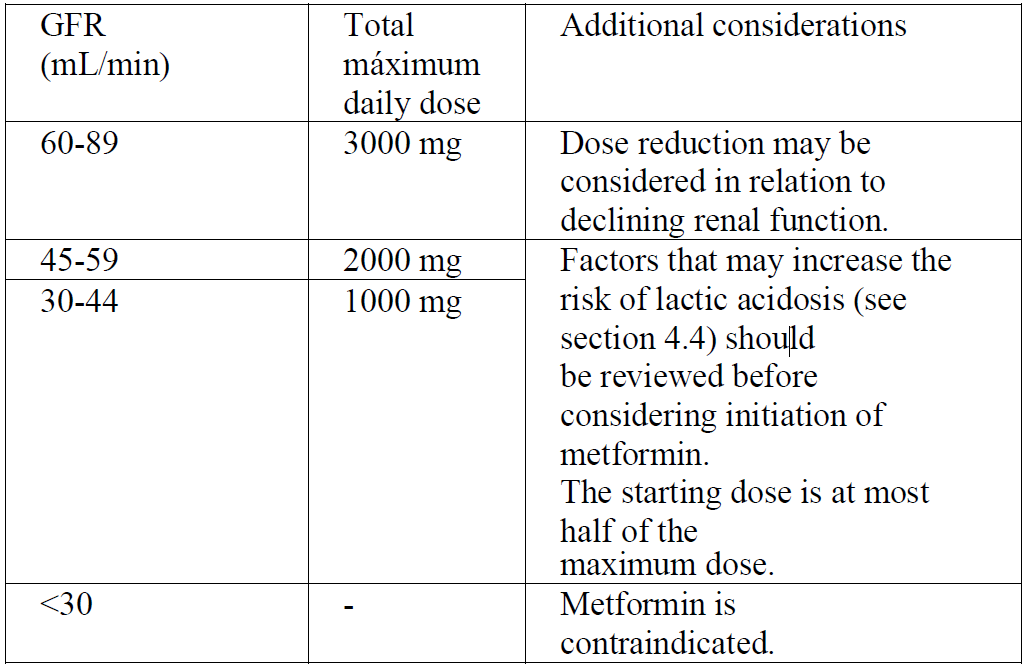 _Paediatric population_ **Monotherapy and combination with insulin** - Metformin can be used in children from 10 years of age and adolescents. - The usual starting dose is 500 mg or 850 mg metformin hydrochloride once daily, given during or after meals. After 10 to 15 days the dose should be adjusted on the basis of blood glucose measurements. A slow increase of dose may improve gastrointestinal tolerability. The maximum recommended dose of metformin hydrochloride is 2 g daily, taken as 2 or 3 divided doses.
ORAL
Medical Information
**4.1. Therapeutic indications** Treatment of type 2 diabetes mellitus, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. - In adults, Metformin may be used as monotherapy or in combination with other oral anti-diabetic agents or with insulin. - In children from 10 years of age and adolescents, Metformin may be used as monotherapy or in combination with insulin. A reduction of diabetic complications has been shown in overweight type 2 diabetic adult patients treated with metformin as first-line therapy after diet failure (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3. Contraindications** - Hypersensitivity to metformin or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. - Any type of acute metabolic acidosis (such as diabetic ketoacidosis, lactic acidosis). - Diabetic pre-coma. - Severe renal failure (GFR <30 mL/min). - Acute conditions with the potential to alter renal function such as: dehydration, severe infection, shock. - Disease which may cause tissue hypoxia (especially acute disease, or worsening of chronic disease) such as: decompensated heart failure, respiratory failure, recent myocardial infarction, shock. - Elective major surgery (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Hepatic insufficiency, acute alcohol intoxication, alcoholism.
A10BA02
metformin
Manufacturer Information
PAN-MALAYAN PHARMACEUTICALS PTE LTD
SAG Manufacturing S.L.U.
Active Ingredients
Documents
Package Inserts
Metforvitae 500mg and 850mg Package Insert_final.pdf
Approved: March 4, 2022
