Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2. Posology and method of administration** Treatment with TALZENNA should be initiated and supervised by a physician experienced in the use of anticancer medicinal products. Detection of mutations in hereditary breast cancer-related BRCA1 and BRCA2 genes should be determined by an experienced laboratory using a validated test method (see Section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Posology The recommended dose of TALZENNA is 1 mg capsule taken orally once daily. Patients should be treated until disease progression or unacceptable toxicity occurs. _Missing dose_ If the patient vomits or misses a dose, an additional dose should not be taken. The next prescribed dose should be taken at the usual time. _Dose modifications_ To manage adverse reactions, consider interruption of treatment or dose reduction based on severity and clinical presentation. Recommended dose reductions are indicated in Table 1. 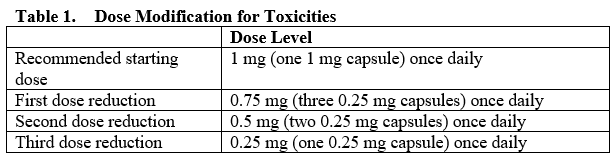 Complete blood count should be obtained prior to starting TALZENNA therapy and monitored monthly and as clinically indicated (see Table 2 and Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 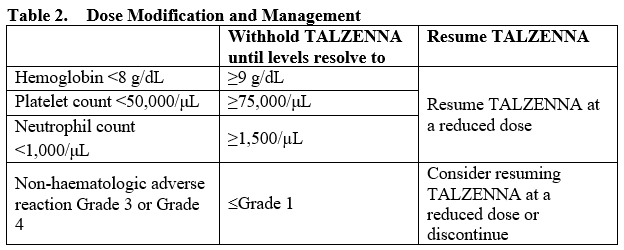 _Concomitant treatment with inhibitors of P-glycoprotein (P-gp)_ Strong inhibitors of P-gp may lead to increased talazoparib exposure. Concomitant use of strong P-gp inhibitors during treatment with talazoparib should be avoided. Coadministration should only be considered after careful evaluation of the potential benefits and risks. If coadministration with a strong P-gp inhibitor is unavoidable, the TALZENNA dose should be reduced to the next lower dose. When the strong P-gp inhibitor is discontinued, the TALZENNA dose should be increased (after 3 to 5 half-lives of the P-gp inhibitor) to the dose used prior to the initiation of the strong P-gp inhibitor (see Section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Concomitant treatment with inhibitors of Breast Cancer Resistance Protein (BCRP)_ The effect of coadministration of BCRP inhibitors with TALZENNA has not been studied. Therefore, concomitant use of strong BCRP inhibitors during treatment with talazoparib should be avoided (see Section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Special populations _Hepatic impairment_ No dose adjustment is required for patients with mild hepatic impairment (total bilirubin ≤1 × upper limit of normal \[ULN\] and aspartate aminotransferase (AST) >ULN, or total bilirubin >1.0 to 1.5 × ULN and any AST), moderate hepatic impairment (total bilirubin >1.5 to 3.0 × ULN and any AST), or severe hepatic impairment (total bilirubin >3.0 × ULN and any AST) (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ No dose adjustment is required for patients with mild renal impairment (60 mL/min ≤ creatinine clearance \[CrCL\] < 90 mL/min). For patients with moderate renal impairment (30 mL/min ≤ CrCL < 60 mL/min), the recommended dose of TALZENNA is 0.75 mg once daily. For patients with severe renal impairment (15 mL/min ≤ CrCL < 30 mL/min), the recommended dose of TALZENNA is 0.5 mg once daily. TALZENNA has not been studied in patients requiring hemodialysis (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Elderly population_ No dose adjustment is necessary in elderly (≥65 years of age) patients (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Pediatric population_ The safety and efficacy of TALZENNA in children and adolescents <18 years of age have not been established.
ORAL
Medical Information
**4.1. Therapeutic indications** TALZENNA is indicated for the treatment of adult patients with germline breast cancer susceptibility gene (BRCA)-mutated human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer who have previously been treated with chemotherapy. These patients could have received chemotherapy in the neoadjuvant, adjuvant, locally advanced or metastatic setting unless patients were not suitable for these treatments.
**4.3. Contraindications** None.
L01XX60
xl 01 xx 60
Manufacturer Information
PFIZER PRIVATE LIMITED
Excella GmbH & Co. KG
Active Ingredients
Documents
Package Inserts
Talzenna Capsule PI and PIL.pdf
Approved: April 18, 2022
