Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**4.2 DOSE AND METHOD OF ADMINISTRATION** ZEPZELCA must be administered under the supervision of a physician experienced in the use of chemotherapy. Its use should be confined to qualified oncologists or other health professionals specialised in the administration of cytotoxic agents. **Recommended Dose and Schedule** The recommended dose is 3.2 mg/m2 by intravenous infusion over 60 minutes repeated every 21 days until disease progression or unacceptable toxicity. Only administer ZEPZELCA to patients with an absolute neutrophil count above 1.5 x 109/L, and a platelet count above 100 x 109/L. **Dose modifications for adverse reactions** The recommended dose reduction levels for adverse reactions are listed in Table 1. Dosage modifications for ZEPZELCA for adverse reactions are presented in Table 2. Permanently discontinue ZEPZELCA in patients who are unable to tolerate 2.0 mg/m2 or require a dose delay greater than two weeks. 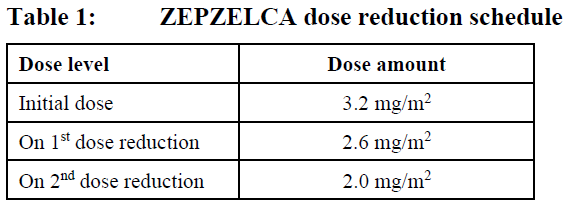 Discontinue ZEPZELCA if patients are unable to tolerate 2.0 mg/m2 every 21 days. 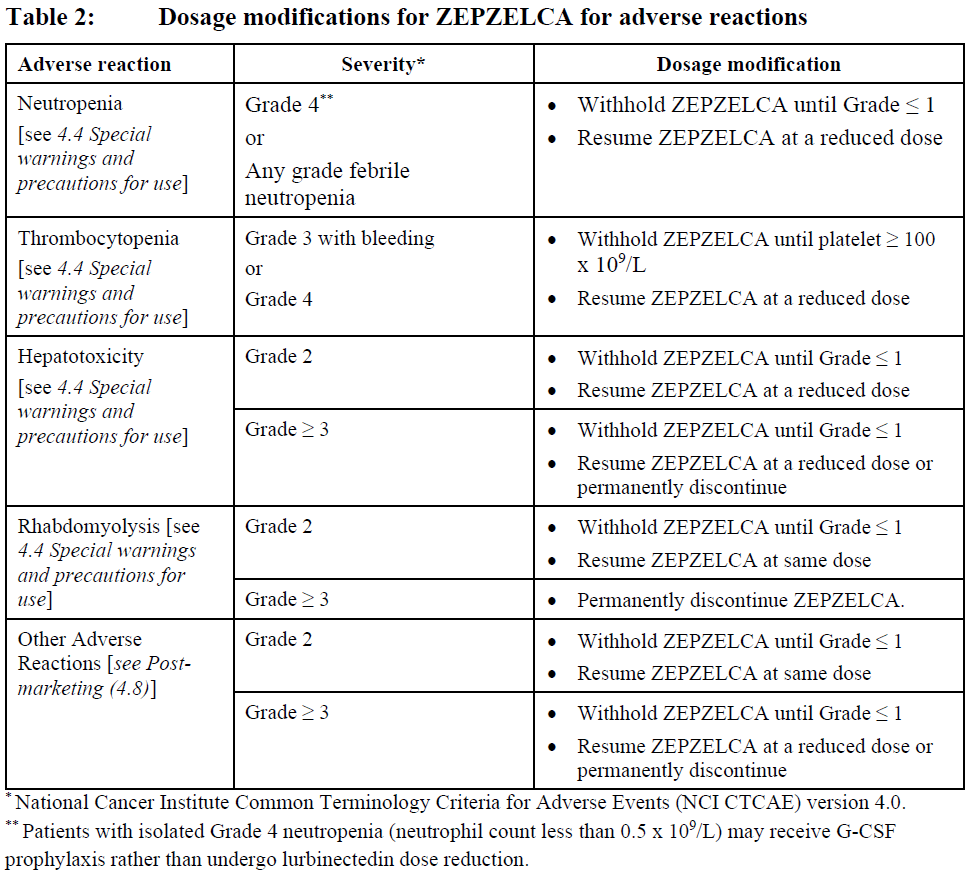 **Premedication** Pre-infusion Medication: Administer the following pre-infusion medications for antiemetic prophylaxis: - Corticosteroids (intravenous dexamethasone 8 mg or equivalent) - Serotonin antagonists (intravenous ondansetron 8 mg or equivalent) Post-infusion Medication: Administer post-infusion medication for extended antiemetic treatment for 2 days after the infusion if needed: - Corticosteroids (oral dexamethasone 4 mg or equivalent) - Serotonin antagonists (oral ondansetron 8 mg or equivalent) or - Metoclopramide (intravenous or oral 10 mg or equivalent every 8 hours) **Preparation** The ZEPZELCA vial is for single-use only. Prepare the solution for infusion using aseptic technique as follows: - Inject 8 mL of Sterile Water for Injection USP into the vial. Shake the vial until complete dissolution. The reconstituted solution is a clear, colourless or slightly yellowish solution, essentially free of visible particles. - Visually inspect the solution for particulate matter and discoloration. Dilute the reconstituted solution with 0.9% Sodium Chloride Injection USP or 5% Dextrose Injection USP. - Calculate the required volume of reconstituted solution as follows: 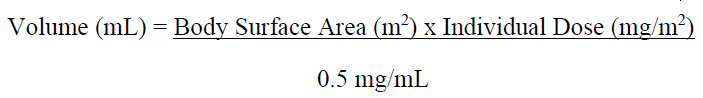 - For administration through a central venous line, withdraw the appropriate amount of reconstituted solution from the vial and add to an infusion container containing at least 100 mL of diluent (0.9% Sodium Chloride Injection USP or 5% Dextrose Injection USP). - For administration through a peripheral venous line, withdraw the appropriate amount of reconstituted solution from the vial and add to an infusion container containing at least 250 mL of diluent (0.9% Sodium Chloride Injection USP or 5% Dextrose Injection USP). - If not used immediately after reconstitution or dilution, the solution can be stored prior to administration for up to 24 hours following reconstitution, including infusion time, at either room temperature/light or under refrigerated (2° to 8° C) conditions. **Administration** - Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter is observed, do not administer. - ZEPZELCA can be administered with or without an in-line filter. If infusion lines containing in-line filters are utilized for administration of ZEPZELCA, Polyethersulfone (PES) in-line filters with pore sizes of 0.22 micron are recommended. - Do not use in-line nylon membrane filters when the reconstituted ZEPZELCA solution is diluted using 0.9% Sodium Chloride Injection, USP. Adsorption of ZEPZELCA to the Nylon membrane filters has been observed when 0.9% Sodium Chloride Injection, USP is used as the diluent. - Compatibility with other intravenous administration materials and the diluted ZEPZELCA solution has been demonstrated in the following materials: - Polyolefin containers (polyethylene, polypropylene and mixtures). - Polyvinyl Chloride (PVC) (non-DEHP-containing), polyurethane and polyolefin infusion sets (polyethylene, polypropylene and polybutadiene). - Implantable venous access systems with titanium and plastic resin ports and with polyurethane or silicone intravenous catheters. - Do not co-administer ZEPZELCA and other intravenous drugs concurrently within the same intravenous line. **Dose Modification for Renal Impairment** - Avoid administration of ZEPZELCA to patients with calculated creatinine clearance less than 30 mL/min. **Dose Modification for Hepatic Impairment** - Do not administer ZEPZELCA to patients with AST or ALT greater than 3 x upper limit of normal (ULN) and/or total bilirubin greater than 1.5 x ULN.
INTRAVENOUS
Medical Information
**4.1 THERAPEUTIC INDICATIONS** ZEPZELCA is indicated for the treatment of adult patients with metastatic small cell lung cancer (SCLC) who have progressed after prior platinum-containing therapy. This indication is approved under provisional approval based on overall response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials \[ _see 5.1 Clinical Trials_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
**4.3 CONTRAINDICATIONS** ZEPZELCA is contraindicated in patients with history of significant drug allergy to the active substance or any of the excipients.
pending
xpending
Manufacturer Information
SPECIALISED THERAPEUTICS ASIA PTE. LTD.
GP–Pharm, S.A.
Baxter Oncology GmbH
Active Ingredients
Documents
Package Inserts
Zepzelca Infusion PI.pdf
Approved: November 16, 2022
