Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and Administration** **Dosage** **_Patients new to methylphenidate_** The recommended starting dosage of CONCERTA® for patients who are not currently taking methylphenidate or stimulants other than methylphenidate is 18 mg once daily for children and adolescents and 18 to 36 mg once daily for adults. **_Patients currently using methylphenidate_** The recommended dosage of CONCERTA® for patients who are currently taking methylphenidate twice daily or three times daily at dosages of 10 to 60 mg/day is provided in Table 1. 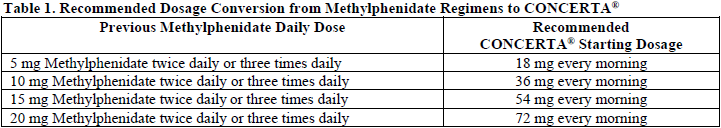 Clinical judgment should be used when selecting the starting dose for patients currently taking methylphenidate in other regimens. **_Dose titration_** The dosage should be individualized according to the needs and responses of the patient. Doses may be increased in 18 mg increments at weekly intervals. Daily dosages above 54 mg in children, 72 mg in adolescents have not been studied and are not recommended. Daily dosages above 72 mg in adults are not recommended. A 27mg dosage strength is available for physicians who wish to prescribe between 18 and 36mg dosage. **_Maintenance/extended treatment_** There is no body of evidence available from controlled trials to indicate how long the patient with ADHD should be treated with CONCERTA®. It is generally agreed, however, that pharmacological treatment of ADHD may be needed for extended periods. Nevertheless, the physician who elects to use CONCERTA® for extended periods in patients with ADHD should periodically re-evaluate the long-term usefulness of the drug for the individual patient with trials off medication to assess the patient’s functioning without pharmacotherapy. Improvement may be sustained when the drug is either temporarily or permanently discontinued. **_Dose reduction and discontinuation_** If paradoxical aggravation of symptoms or other adverse events occur, the dosage should be reduced, or, if necessary, the drug should be discontinued. If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued. **Administration** CONCERTA® is administered orally once daily. As the effect has been shown to be present 12 hours after dosing, the product should be taken once daily in the morning. CONCERTA® must be swallowed whole with the aid of liquids, and must not be chewed, divided, or crushed (see _Precautions – Information for Patients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). CONCERTA® may be administered with or without food (see _Pharmacokinetic Properties – Food effects_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Special populations** **_Pediatrics (under 6 years of age)_** Use of CONCERTA® in patients under six years of age has not been studied in controlled trials. CONCERTA® should not be used in patients under six years old. Long-term effects of methylphenidate in children are not yet available. Although a causal relationship has not been established, suppression of growth (ie weight gain and/or height) has been reported with the long-term use of stimulants in children. Therefore, patients requiring long-term therapy should be carefully monitored. Patients who are not growing or gaining weight as expected should have their treatment interrupted. **_Elderly (over 65 years of age)_** Use of CONCERTA® in elderly patients over 65 years of age has not been studied in controlled trials. **_Renal insufficiency_** There is no experience with the use of CONCERTA® in patients with renal insufficiency (see _Pharmacokinetic Properties – Special populations, Renal insufficiency_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **_Hepatic insufficiency_** There is no experience with the use of CONCERTA® in patients with hepatic insufficiency.
ORAL
Medical Information
**Indications** CONCERTA® is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). The efficacy of CONCERTA® in the treatment of ADHD was established in controlled trials of children and adolescents aged 6 to 17 and adults aged 18 to 65 who met DSM-IV criteria for ADHD. A diagnosis of Attention Deficit Hyperactivity Disorder (ADHD; DSM-IV) implies the presence of hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, eg, in social, academic, or occupational functioning, and be present in two or more settings, eg, school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive type, at least six of the following symptoms must have persisted for at least 6 months, lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least six of the following symptoms must have persisted for at least 6 months; fidgeting/squirming; leaving seat; inappropriate running/climbing; difficult with quiet activities; “on the go”; excessive talking; blurting answers; can’t wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met. **Special Diagnostic Considerations** Specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use of medical and special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the child and not solely on the purpose of the required number of DSM-IV characteristics. **Need for Comprehensive Treatment Program** CONCERTA® is indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all children with this syndrome. Stimulants are not intended for use in the child who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician’s assessment of the chronicity and severity of the child’s symptoms. **Long-Term Use** The effectiveness of CONCERTA® for long-term use, ie, for more than 4 weeks, has not been systematically evaluated in controlled trials. Therefore, the physician who elects to use CONCERTA® for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (see _Dosage and Administration_).
**Contraindications** CONCERTA® is contraindicated: - in patients known to be hypersensitive to methylphenidate or other components of the product; - in patients with glaucoma; - during treatment with monoamine oxidase (MAO) inhibitors, and also within a minimum of 14 days following discontinuation of a monoamine oxidase (MAO) inhibitor (hypertensive crisis may result) (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_); - in patients with hyperthyroidism; - in patients with severe angina pectoris; - in patients with cardiac arrhythmia; - in patients with phaeochromocytoma.
N06BA04
methylphenidate
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
JANSSEN-CILAG MANUFACTURING, LLC
Active Ingredients
Documents
Package Inserts
Concerta Extended-Release Tablet PI.pdf
Approved: April 13, 2023
