Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**DOSAGE AND ADMINISTRATION** _Film-coated tablets_ The film-coated tablets must be taken orally, swallowed with a sufficient quantity of liquid and may be taken with or without food. After oral administration the bitter taste of levetiracetam may be experienced. The daily dose is administered in two equally divided doses. Purpose of the breakline is to divide tablet into equal halves. **Route of Administration** For oral use: Levetiracetam Tablets 250 mg and 500 mg **Adults** - Monotherapy Adults and adolescents from 16 years of age The recommended starting dose is 250 mg twice daily which should be increased to an initial therapeutic dose of 500 mg twice daily after two weeks. The dose can be further increased by 250 mg twice daily every two weeks depending upon the clinical response. The maximum dose is 1500 mg twice daily. - Add-on therapy Adults (≥18 years) and adolescents (12 to 17 years) of 50 kg or more The initial therapeutic dose is 500 mg twice daily. This dose can be started on the first day of treatment. Depending upon the clinical response and tolerance, the daily dose can be increased up to 1,500 mg twice daily. Dose changes can be made in 500 mg twice daily increases or decreases every two to four weeks. **Children** The tablet formulation is not adapted for use in children under the age of 6 years. Levetiracetam oral solution is the preferred formulation for use in this population. In addition, the available dose strengths of the tablets are not appropriate for initial treatment in children weighing less than 25 kg, for patients unable to swallow tablets or for the administration of doses below 250 mg. In all of the above cases, levetiracetam oral solution should be used. Monotherapy The safety and efficacy of levetiracetam in children and adolescents below 16 years as monotherapy treatment have not been established. There are no data available. Add-on Therapy for Children (4 to 11 years) and Adolescents (12 to 17 years) weighing less than 50 kg For children 6 years and above, levetiracetam oral solution should be used for doses under 250 mg, for doses not multiple of 250 mg when dosing recommendation is not achievable by taking multiple tablets and for patients unable to swallow tablets. The physician should prescribe the most appropriate strength according to age, weight and dose. The initial therapeutic dose is 10 mg/kg twice daily. Depending upon the clinical response and tolerability, the dose can be increased up to 30 mg/kg twice daily. Dose changes should not exceed increases or decreases of 10 mg/kg twice daily every two weeks. The lowest effective dose should be used. Dose in children 50 kg or greater is the same as in adults. 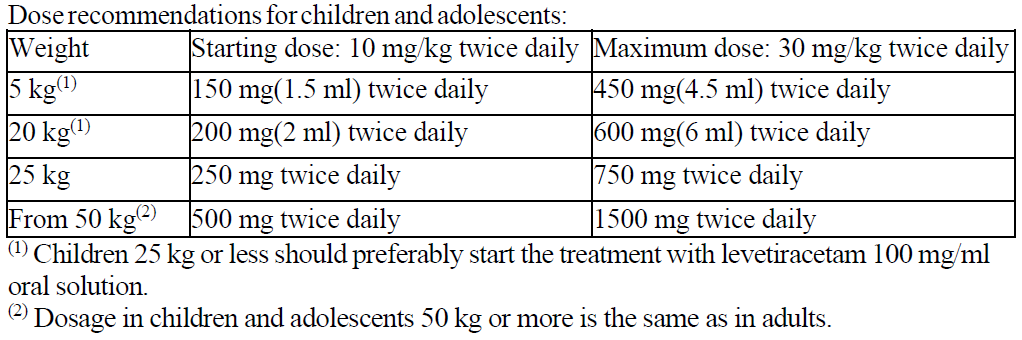 Infants and children less than 4 years There are insufficient data to recommend the use of Levetiracetam in children under 4 years of age. Elderly Adjustment of the dose is recommended in elderly patients with compromised renal function. Renal impairment The daily dose must be individualized according to renal function ( _see section Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For adult patients, refer to the following table and adjust the dose as indicated. To use this dosing table, an estimate of the patient's creatinine clearance (CLcr) in ml/min is needed. The CLcr in ml/min may be estimated from serum creatinine (mg/dl) determination, for adults and adolescents weighing 50 kg or more, using the following formula: 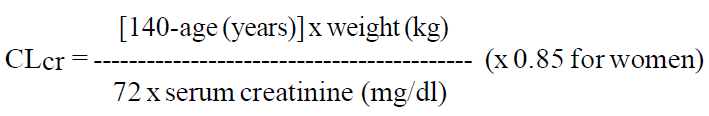 Then CLcr is adjusted for body surface area (BSA) as follows: 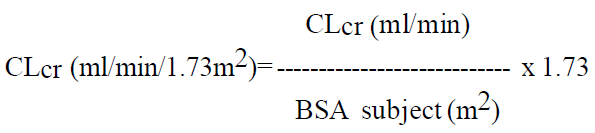 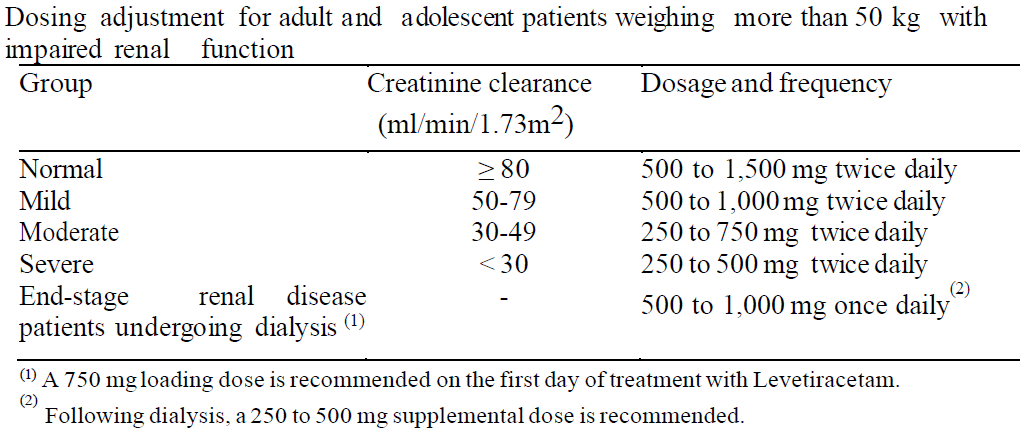 For children with renal impairment, Levetiracetam dose needs to be adjusted based on the renal function as Levetiracetam clearance is related to renal function. This recommendation is based on a study in adult renally-impaired patients. Hepatic impairment No dose adjustment is needed in patients with mild to moderate hepatic impairment. In patients with severe hepatic impairment, the creatinine clearance may underestimate the renal insufficiency. Therefore, a 50% reduction of the daily maintenance dose is recommended when the creatinine clearance is <60 ml/min/1.73m2 ( _see section Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**INDICATIONS** Levetiracetam is indicated as monotherapy in the treatment of partial onset seizures with or without secondary generalisation in adults and adolescents from 16 years of age with newly diagnosed epilepsy. Levetiracetam is indicated as adjunctive therapy in the treatment of: - partial onset seizures with or without secondary generalisation in adults, adolescents and children from 4 years of age with epilepsy. - myoclonic seizures in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy. - primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with Idiopathic Generalised Epilepsy.
**CONTRAINDICATIONS** Hypersensitivity to the active substance or other pyrrolidone derivatives or to any of the excipients.
N03AX14
levetiracetam
Manufacturer Information
ACCORD HEALTHCARE PRIVATE LIMITED
Intas Pharmaceuticals Limited
Active Ingredients
Documents
Package Inserts
Levera Tablets PI.pdf
Approved: May 22, 2023
