Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**DOSAGE AND ADMINISTRATION** AERRANE (isoflurane) should be administered only by persons trained in the administration of general anaesthesia. Facilities for maintenance of a patent airway, artificial ventilation, oxygen enrichment, and circulatory resuscitation must be immediately available. AERRANE (isoflurane) is administered by inhalation. AERRANE (isoflurane) should be delivered from a vaporiser specifically designed for use with isoflurane. Dosage for induction and maintenance must be individualised and titrated to the desired effect according to the patient’s age and clinical status. The need for premedication and type of premedicants must be determined on an individual basis. With the exception of neonates, the minimum alveolar concentration (MAC) of AERRANE (isoflurane) decreases with increasing patient age. AERRANE (isoflurane) MAC values according to age as shown below: 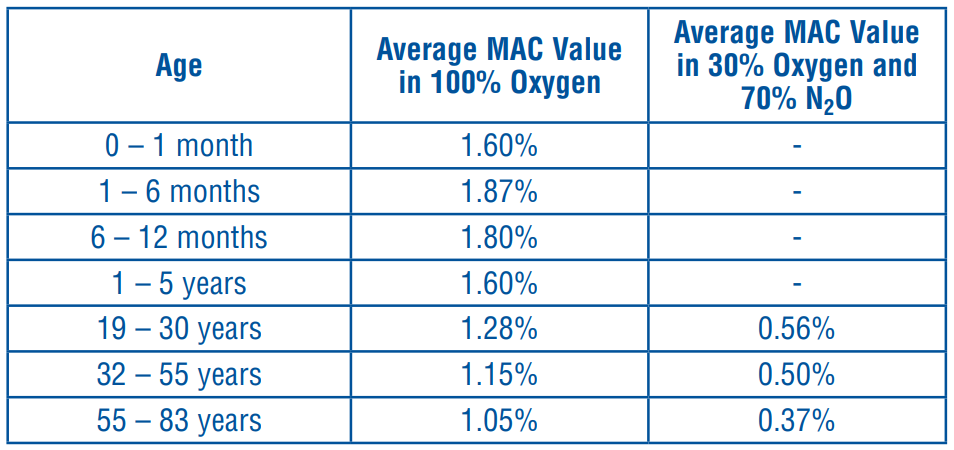 **Induction of Anaesthesia** If AERRANE (Isoflurane) is used for induction of anaesthesia, a starting concentration of 0.5% is recommended. Concentrations of 1.3 – 3.0% usually bring about surgical anaesthesia within 7 to 10 minutes. It is recommended that use be made of a hypnotic dose of a short acting barbiturate or another product such as propofol, etomidate, or midazolam in order to avoid coughing or laryngospasm, which can arise if induction is carried out with AERRANE (isoflurane) alone or in combination with oxygen or with an oxygen-nitrous oxide mixture. When using AERRANE (isoflurane) for induction, it should be considered that the risk of coughing, breath holding, laryngospasm, and bronchospasm during induction increases with the concentration of AERRANE (isoflurane). **Maintenance of Anaesthesia** Anaesthesia can be maintained during surgery using a concentration of 1.0 – 2.5%, with the simultaneous administration of N2O and O2. A higher concentration of 1.5 – 3.5% of AERRANE (isoflurane) is necessary if AERRANE (isoflurane) is administered with pure oxygen. **Recovery** The concentration of AERRANE (isoflurane) must be reduced to 0.5% at the end of the operation, or to 0% during closure of the wound to allow prompt recovery. If all administration of anaesthetic agents has been stopped, the air passages of the patient should be ventilated several times with 100% oxygen until complete awakening occurs. If the vector gas is a mixture of 50% O2 and 50% N2O, the volume of the minimum alveolar concentration of AERRANE (isoflurane) is approximately 0.65%.
NASAL
Medical Information
**INDICATIONS** AERRANE (isoflurane) is a volatile halogenated anaesthetic for general inhalation anaesthesia.
**CONTRAINDICATIONS** AERRANE (isoflurane) is contraindicated in patients: - with known hypersensitivity to AERRANE (isoflurane) or to other halogenated inhalational anaesthetics - with known or suspected susceptibility to malignant hyperthermia - with a history of confirmed hepatitis due to halogenated inhalational anaesthetic or a history of unexplained moderate to severe hepatic dysfunction (e.g., jaundice associated with fever and/or eosinophilia) after anaesthesia with AERRANE (isoflurane) or other halogenated inhalational anaesthetics. - Obstetric operation - Nonselective MAOI (See Interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - in whom general anaesthesia is contraindicated.
N01AB06
isoflurane
Manufacturer Information
BAXTER HEALTHCARE (ASIA) PTE LTD
Baxter Healthcare Corporation
Active Ingredients
Documents
Package Inserts
Aerrane Inhalation PI.pdf
Approved: February 7, 2022
