Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**2.2 DOSAGE AND ADMINISTRATION** **General** Substitution by any other biological medicinal product requires the consent of the prescribing physician. The safety and efficacy of alternating or switching between Enspryng and products that are biosimilar but not deemed interchangeable have not been established. Therefore, the benefit-risk of alternating or switching needs to be carefully considered. In order to prevent medication errors, it is important to check the prefilled syringe label to ensure that the drug being administered is Enspryng. **Recommended Dosage** Enspryng must be administered as a subcutaneous injection. Advise patients to consult with their healthcare professional (HCP) if they suspect an active infection (including localized infections) before administration or the next dose of Enspryng. In case of active infection, delay use of Enspryng until the infection is controlled ( _see section 2.4 Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Enspryng can be used as a monotherapy or in combination with either oral corticosteroids (OCs), azathioprine (AZA) or mycophenolate mofetil (MMF) ( _see section 3.1.2 Clinical / Efficacy Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Please also refer to the full prescribing information for these products. _Loading Dose_ The recommended loading dose is 120 mg SC injection every 2 weeks (first dose at week 0, second dose at week 2 and third dose at week 4) for the first three administrations. _Maintenance Dose_ The recommended maintenance dose is 120 mg SC injection every 4 weeks. **Method of administration** The recommended injection sites are the abdomen and thigh. Injection sites should be rotated, and injections should never be given into moles, scars, or areas where the skin is tender, bruised, red, hard, or not intact. Comprehensive instructions for the administration of Enspryng are given in the Instructions for Use (IFU). The first injection must be performed under the supervision of a qualified healthcare professional (HCP). After adequate training on how to prepare and perform the injection, an adult patient/caregiver may administer Enspryng at home if the treating physician determines that it is appropriate and the adult patient/caregiver can perform the injection technique. Patients/caregivers should seek immediate medical attention if the patient develops symptoms of serious allergic reactions and should check with their HCP to confirm whether treatment with Enspryng can be continued or not. **Duration of Treatment** Enspryng is intended for long-term treatment. **Delayed or Missed Doses** If an injection is missed, for any reason other than increases in liver enzymes, it should be administered as described in Table 1. 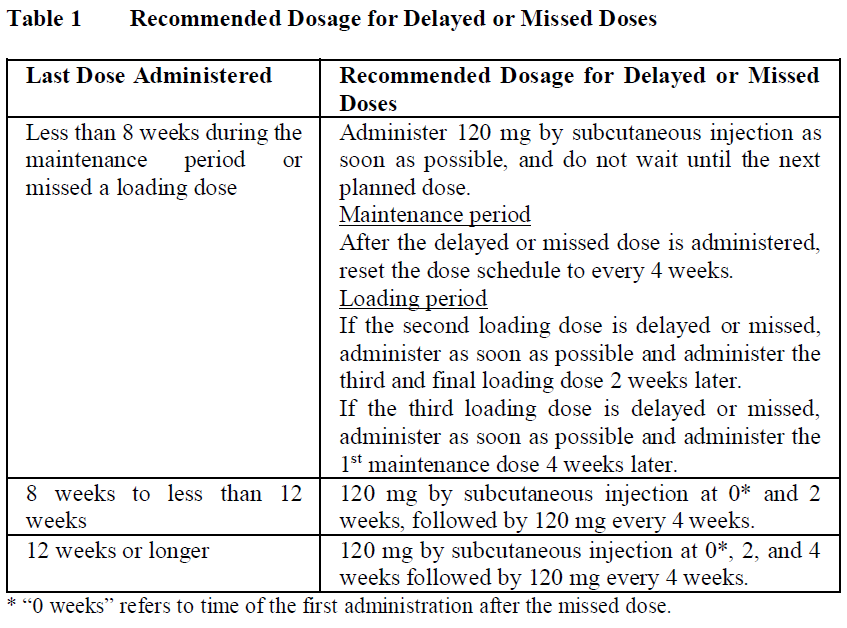 **Dose Modifications** _Liver Enzyme Abnormalities_ If the alanine aminotransferase (ALT) or aspartate transaminase (AST) elevation is >5x Upper Limit of Normal (ULN) and associated with any bilirubin elevation, treatment with Enspryng must be discontinued, and reinitiation is not recommended. If the ALT or AST elevation is >5x ULN and not associated with any bilirubin elevation, treatment with Enspryng should be discontinued; it can be restarted (120 mg SC injection every 4 weeks) when the ALT and AST levels have returned to the normal range and based on assessment of benefit-risk of treatment in the patient. If the decision is taken to restart treatment, the liver parameters must be closely monitored, and if any subsequent increase in ALT/AST and/or bilirubin is observed, the drug must be discontinued, and reinitiation is not recommended. 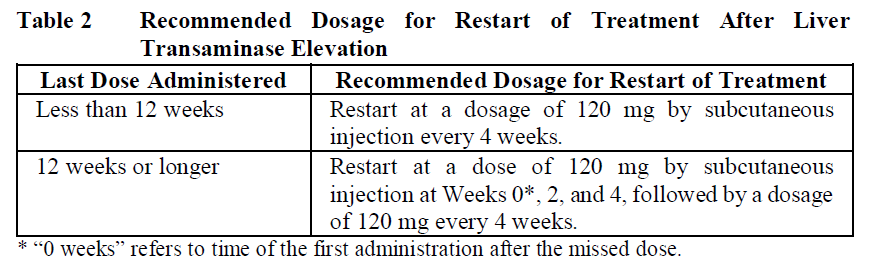 _Neutropenia_ If the neutrophil count is below 1.0 x109/L and confirmed by repeat testing, Enspryng should be interrupted until the neutrophil count is > 1.0 x109/L. **2.2.1 Special Dosage Instructions** **Pediatric use** The posology in adolescent patients ≥12 years of age with body weight ≥ 40 kg and adult patients is the same. The safety and efficacy of Enspryng in pediatric population <12 years of age have not been studied ( _see section 2.5.4 Pediatric Use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Geriatric use** No dose adjustment is required in patients ≥65 years of age ( _see sections 2.5.5 Geriatric Use and 3.2.5 Pharmacokinetics in special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal Impairment** The safety and efficacy of Enspryng have not been formally studied in patients with renal impairment; however, a dose adjustment is not expected to be required for patients with renal impairment ( _see sections 2.5.6 Renal Impairment and 3.2.5 Pharmacokinetics in special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic Impairment** The safety and efficacy of Enspryng have not been studied in patients with hepatic impairment ( _see sections 2.5.7 Hepatic Impairment and 3.2.5 Pharmacokinetics in special populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Other Special Patient Populations** Not applicable
SUBCUTANEOUS
Medical Information
**2.1 THERAPEUTIC INDICATION(S)** Enspryng is indicated as a monotherapy or in combination with immunosuppressive therapy (IST) for the treatment of neuromyelitis optica spectrum disorders (NMOSD) in adult and adolescents who are anti-aquaporin 4 (AQP4) seropositive.
**2.3 CONTRAINDICATIONS** Enspryng is contraindicated in patients with a known hypersensitivity to satralizumab or any of the excipients.
L04AC19
satralizumab
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Chugai Pharma Manufacturing Co., Ltd. (Bulk production and Primary packaging)
Active Ingredients
Documents
Package Inserts
ENSPRYNG SOLUTION FOR INJECTION IN PREFILLED SYRINGE PI.pdf
Approved: October 19, 2021
