Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED, EXTENDED RELEASE
**Dosage and Administration** **Pharmaceutical form** Film-coated, capsule-shaped tablets for oral administration. The tablet strengths are distinguished by colour and debossing. 2 mg: pink, capsule-shaped, film-coated tablets marked “GS” on one side and “3V2” on the other. 4 mg: light brown, capsule-shaped, film-coated tablets marked “GS” on one side and “WXG” on the other. When switching treatment from another dopamine agonist to _REQUIP PD 24 HOUR_, the manufacturer’s guidance on discontinuation should be followed before initiating _REQUIP PD 24 HOUR_. Individual dose titration against efficacy and tolerability is recommended. Patients should be down-titrated if they experience disabling somnolence at any dose level. For other adverse events, down-titration followed by more gradual up-titration has been shown to be beneficial. - **Adults** _REQUIP PD 24 HOUR_ should be taken as a single daily dose and should be taken at a similar time each day. The tablet(s) must be swallowed whole, and must not be chewed, crushed or divided. _REQUIP PD 24 HOUR_ may be taken with or without food (see _Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Treatment initiation as adjunct to L-dopa** The dose should be titrated according to the individual clinical response and tolerability. The starting dose is 2 mg taken once daily for 1 to 2 weeks, followed by increases of 2 mg/day at 1-week or longer intervals as appropriate, depending on therapeutic response and tolerability, up to a maximally recommended dose of 24 mg/day. Patients should be assessed for therapeutic response and tolerability at a minimal interval of 1 week or longer after each dose increment. Caution should be exercised during dose titration because too rapid a rate of titration may lead to dose selection that may not provide additional benefit, but that may increase the risk of adverse reactions. The safety and efficacy of doses above 24 mg/day have not been established. It may be possible to reduce gradually the L-dopa dose, depending on the clinical response. In clinical trials, the L-dopa dose was reduced gradually once the patient reached a dose of _REQUIP PD 24 HOUR_ of 8 mg/day. On average, the L-dopa dose was reduced by approximately 30% in patients receiving _REQUIP PD 24 HOUR_ as compared to approximately 20% in patients treated with placebo. In patients with advanced Parkinson’s disease receiving _REQUIP PD 24 HOUR_ in combination with L-dopa, dyskinesias can occur during the initial titration of _REQUIP PD 24 HOUR_ (see _Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Sudden discontinuation of _REQUIP PD 24 HOUR_ is not recommended. _REQUIP PD 24 HOUR_ should be discontinued gradually over a period of 7 days under the advice of the doctor (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If treatment is interrupted for one day or more, re-initiation by dose titration should be considered (see above). **Switching from ropinirole immediate release tablets to ropinirole prolonged release tablets** Patients may be switched overnight from _REQUIP_ immediate release (IR) tablets to _REQUIP PD 24 HOUR_ PR tablets. The dose of _REQUIP PD 24 HOUR_ PR tablets should be based on the total daily dose of _REQUIP_ IR tablets that the patient was taking. If patients are taking a different total daily dose of ropinirole immediate release tablets to those typically prescribed doses as shown in the table, they should be switched to the nearest available dose of ropinirole prolonged release tablets as stated in the table. The table below shows the recommended dose of _REQUIP PD 24 HOUR_ PR tablets for patients switching from _REQUIP_ IR tablets: 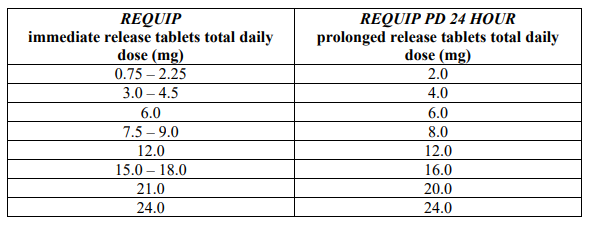 After switching to _REQUIP PD 24 HOUR_ PR tablets, the dose may be adjusted depending on the therapeutic response (see “ _Treatment initiation_” and “ _Therapeutic regimen_” above). - **Elderly** The clearance of ropinirole is decreased in patients aged 65 years or above, but the dose of _REQUIP PD 24 HOUR_ for elderly patients can be titrated in the normal manner based on therapeutic responses and tolerability. - **Children and Adolescents** The safety and efficacy of ropinirole have not been established in patients under 18 years of age; therefore, _REQUIP PD 24 HOUR_ is not recommended for use in patients within this age group. - **Renal impairment** In patients with mild to moderate renal impairment (creatinine clearance 30 – 50 mL/min) no change in the clearance of ropinirole was observed, indicating that no dosage adjustment is necessary in this population. A study into the use of ropinirole in patients with end stage renal disease (patients on haemodialysis) has shown that a dose adjustment in these patients is required as follows: The recommended initial dose of _REQUIP PD 24 HOUR_ is 2 mg once daily. Further dose escalations should be based on tolerability and efficacy. The recommended maximum dose is 18 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of ropinirole in patients with severe renal impairment (creatinine clearance less than 30 mL/min) without regular dialysis has not been studied. - **Hepatic impairment** The use of ropinirole in patients with hepatic impairment has not been studied. Administration of _REQUIP PD 24 HOUR_ to such patients is not recommended.
ORAL
Medical Information
**Indications** _REQUIP PD 24 HOUR_ is indicated for the treatment of Parkinson’s disease under the following conditions: - As monotherapy (without L-dopa) to substitute _REQUIP_ immediate release tablet in PD patients already taking _REQUIP_ immediate release tablet whom adequate symptomatic control has been established. - In combination with levodopa, over the course of the disease, when the effect of levodopa wears off or becomes inconsistent and fluctuations in the therapeutic effect occur (“end of dose” or “on-off” type fluctuations).
**Contraindications** Hypersensitivity to ropinirole or to any of the excipients.
N04BC04
ropinirole
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
Glaxo Wellcome S.A.
Active Ingredients
Documents
Package Inserts
Requip PD Tablet PI.pdf
Approved: February 2, 2021
