Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage instructions and method of administration** The dosage is dependent on the patient's immune status and on the severity of the disease. The following dosage suggestions may be used as reference: 1. **Neonates and infants:** 5 ml (0.25 g)/kg body weight daily on 3 consecutive days. Further infusions may be required depending on the clinical course. 2. **Children and adults:** 1. Therapy of severe bacterial infections: 5 ml (0.25 g)/kg body weight daily on 3 consecutive days. Further infusions may be required depending on the clinical course. 2. Immunoglobulin substitution in immunocompromized patients: 3 – 5 ml (0.15 – 0.25 g)/kg body weight. Repetition at weekly intervals if necessary. Pentaglobin should be infused intravenously at the following rates: in neonates and infants:1.7 ml/kg/hour by infusion pumpin children and adults:0.4 ml/kg/hour**alternatively:**the first 100 ml at 0.4 ml/kg/hourthen 0.2 ml/kg/hour continuouslyuntil 15 ml/kg is reached within 72 hours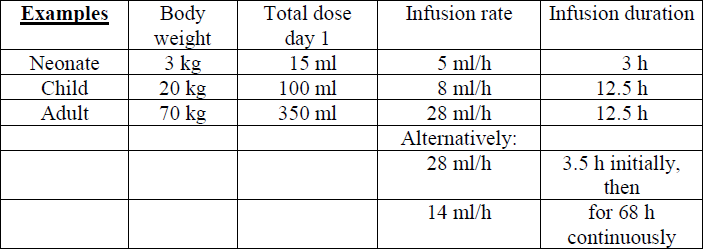 **Method of administration** Intravenous use. Pentaglobin should be brought to room or body temperature before use.
INTRAVENOUS
Medical Information
**Indications** Adjuvant therapy of severe bacterial infections additional to antibiotic therapy. Product is only intended for use in high risk patients with proven bacterial sepsis and who are unlikely to have underlying IgA deficiency. Immunoglobulin substitution in immunocompromised patients.
**Contraindications** Hypersensitivity to the active substance or to any of the excipients listed above – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Hypersensitivity to human immunoglobulins, especially in patients with antibodies against IgA.
J06BA02
immunoglobulins, normal human, for intravascular adm.
Manufacturer Information
GRIFOLS ASIA PACIFIC PTE. LTD.
BIOTEST AG
Active Ingredients
Documents
Package Inserts
Pentaglobin Injection 50mg per mL PI.pdf
Approved: January 3, 2019
