Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**4.2 Dose and method of administration** **Dosage** The dosage recommendations should be strictly adhered to in order to minimise the possibility of dystonic side effects. Metoclopramide should only be used after careful examination has excluded any underlying disorder (such as cerebral irritation) that may have induced nausea and vomiting. For adults and children, the maximum dose in 24 hours is 0.5 mg per kg body weight with a maximum of 30 mg daily. Maximum recommended treatment duration is 5 days in all age groups. The solution can be administered intravenously (IV) or intramuscularly (IM). _**Adults**_ The recommended single dose is 10 mg, repeated up to three times daily. Prevention of PONV: a single dose of 10 mg is recommended. Total daily doses of metoclopramide should not exceed 0.5 mg/kg bodyweight with a maximum of 30 mg daily. The treatment duration should be as short as possible and switch to oral treatment should be made as soon as possible. Treatment durations beyond 12 weeks should be avoided unless the therapeutic benefit is judged to outweigh the risk to the patient. Please refer to dosing in Children section, below. _**Children (aged 1–18 years)**_ The recommended dose is 0.10 to 0.15 mg/kg body weight, repeated up to three times daily by the intravenous route. The recommended maximum dose in 24 hours is 0.5 mg/kg body weight. **Dosing table** 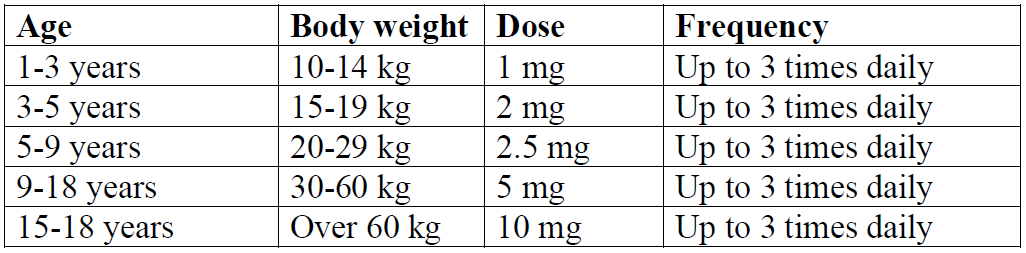 The injectable treatment duration should be as short as possible. The recommended maximum treatment duration is 48 hours for treatment of established post-operative nausea and vomiting (PONV). Metoclopramide is contraindicated in children aged less than 1 year (see Section 4.3 Contraindications). Due to the potential risk of severe cardiovascular reactions including cardiac arrest, the solutions for injection are restricted to be used only when appropriate resuscitation equipment is available (see Section 4.8 Adverse effects (undesirable effects), Cardiovascular – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of administration** IV doses should be administered as a slow bolus (at least over 3 minutes). A minimum interval of 6 hours between two administrations is to be respected, even in case of vomiting or rejection of dose. **Dosage adjustment** **Use in special patient populations** _**Elderly patients**_ In elderly patients, a dose reduction should be considered, based on renal and hepatic function and overall frailty. _**Patients with renal impairment**_ In patients with severe renal impairment (creatinine clearance ≤15 mL/min), the daily dose should be reduced by 75%. In patients with moderate to severe renal impairment (creatinine clearance 15–60 mL/min), the dose should be reduced by 50%. _**Patients with hepatic impairment**_ Metoclopramide undergoes hepatic metabolism via simple conjugation. Its safe use has been described in patients with advanced liver disease whose renal function was normal. In patients with severe hepatic impairment, the dose should be reduced by 50%, with subsequent dose adjustment being made as the individual response has been determined. **Compatibility** **_Intravenous fluids_** No preservative is included in the formulation of Metoclopramide Injection. Therefore, to reduce microbiological hazard, admixture to intravenous fluids should be performed under aseptic conditions and the infusion commenced as soon as possible after preparation and in any case within 24 hours of preparation. If storage is necessary, keep at 2°C to 8°C. The literature indicates that Metoclopramide Injection may be added to the following solutions: - Glucose 5% in sodium chloride 0.45% - Glucose 5% in water - Mannitol 20% - Sodium chloride 0.9% - Ringer’s injection - Ringer’s injection, lactated (Hartmann’s solution) _**Narcotic analgesics**_ - Morphine sulfate: 1 mg/mL with metoclopramide hydrochloride 0.2 mg/mL in glucose 5% in water visually compatible for a 4-hour study period at 25°C under fluorescent light. - Pethidine hydrochloride: 10 mg/mL with metoclopramide hydrochloride 0.2 mg/mL in glucose 5% in water visually compatible for a 4-hour study period at 25°C under fluorescent light. **_Cytotoxic drugs_** If the standard formulation of metoclopramide is used for the treatment of nausea and vomiting associated with cytotoxic drugs, the cytotoxic agent should be administered as a separate infusion.
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**4.1 Therapeutic indications** **Adults** - Prevention of nausea and vomiting associated with chemotherapy and radiotherapy with low and minimal emetogenicity - Prevention of post-operative nausea and vomiting (PONV) - Symptomatic treatment of acute migraine induced nausea and vomiting - Adjuvant to surgical and radiological procedures **Children (aged 1–18 years)** Treatment of established post-operative nausea and vomiting (PONV) as a second line option (intravenous use only).
**4.3 Contraindications** - Patients in whom increased gastrointestinal motility might be dangerous, e.g. presence of gastrointestinal haemorrhage, mechanical obstruction or perforation - Phaeochromocytoma due to the possibility of a hypertensive crisis, probably due to release of catecholamines from the tumour - Known hypersensitivity or intolerance to metoclopramide. Note: patients sensitive to procaine and procainamide may be sensitive to metoclopramide - Patients with porphyria - Metoclopramide should not be used in patients with epilepsy since it may increase the frequency and severity of seizures - Metoclopramide should not be administered to patients receiving other drugs which are likely to cause extrapyramidal reactions, since the frequency and severity of extrapyramidal reactions may be increased - Metoclopramide should not be used in children below 1 year of age - Parkinson’s disease
A03FA01
metoclopramide
Manufacturer Information
PFIZER PRIVATE LIMITED
PFIZER (PERTH) PTY LTD
Active Ingredients
Documents
Package Inserts
Metoclopramide Injection BP PI.pdf
Approved: May 11, 2022
