Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4 Dosage regimen and administration** Treatment with trametinib should only be initiated and supervised by a physician experienced in the administration of anti-cancer medicinal products. Before taking trametinib, patients must have confirmation of BRAF V600 mutation using a validated test. When Mekinist is used in combination with Tafinlar, please also refer to the full Tafinlar Package Insert. **Dosage regimen** **General target population** **Adults** _Recommended Dosage for Unresectable or Metastatic Melanoma_ The recommended dose of trametinib, either used as monotherapy or in combination with dabrafenib, is 2 mg once daily. The recommended dose of dabrafenib, when used in combination with trametinib, is 150 mg twice daily. It is recommended that patients continue treatment with trametinib until patients no longer derive benefit or the development of unacceptable toxicity. _Recommended Dosage for the Adjuvant Treatment of Melanoma_ The recommended dose of trametinib in combination with dabrafenib is 2 mg once daily. The recommended dose of dabrafenib, when used in combination with trametinib, is 150 mg twice daily, until disease recurrence or unacceptable toxicity for up to 1 year. _Recommended Dosage for NSCLC_ The recommended dose of trametinib in combination with dabrafenib, is 2 mg once daily. The recommended dose of dabrafenib, when used in combination with trametinib, is 150 mg twice daily. It is recommended that patients continue treatment with trametinib until patients no longer derive benefit or the development of unacceptable toxicity. _Recommended Dosage for ATC_ The recommended dose of trametinib in combination with dabrafenib, is 2 mg once daily. The recommended dose of dabrafenib, when used in combination with trametinib, is 150 mg twice daily, until disease recurrence or unacceptable toxicity. _Missed doses_ If a dose of trametinib is missed, it should only be taken if it is more than 12 hours until the next scheduled dose. If a dose of dabrafenib is missed, when trametinib is given in combination with dabrafenib, the dose of dabrafenib should only be taken if it is more than 6 hours until the next scheduled dose. **Dose adjustments** **Mekinist as monotherapy and in combination with Tafinlar** The management of adverse events/adverse drug reactions may require treatment interruption, dose reduction, or treatment discontinuation. Dose modifications are not recommended for adverse reactions of cutaneous squamous cell carcinoma (cuSCC) or new primary melanoma (see dabrafenib Package Insert for further details). Recommended dose level reductions are provided in Table 4-1. Doses below 1 mg once daily are not recommended. 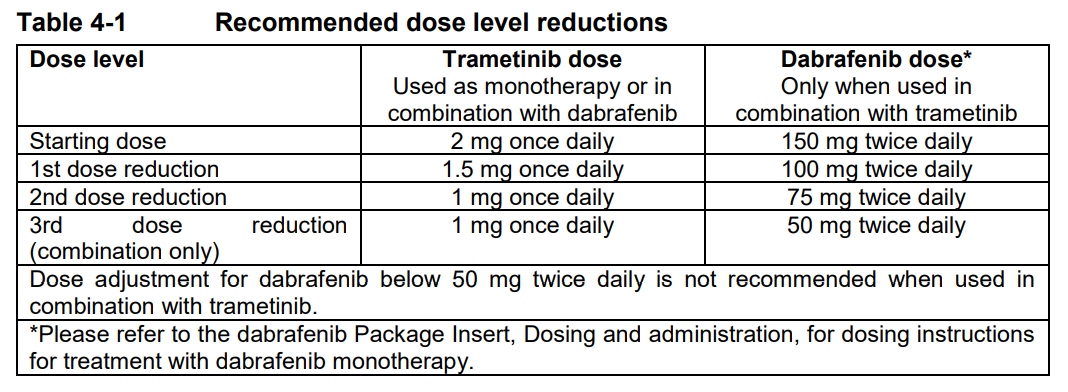 The recommended dose modification schedule is provided in Table 4-2. When an individual’s adverse reactions are under effective management, dose re-escalation following the same dosing steps as de-escalation may be considered. The Mekinist dose should not exceed 2 mg once daily. 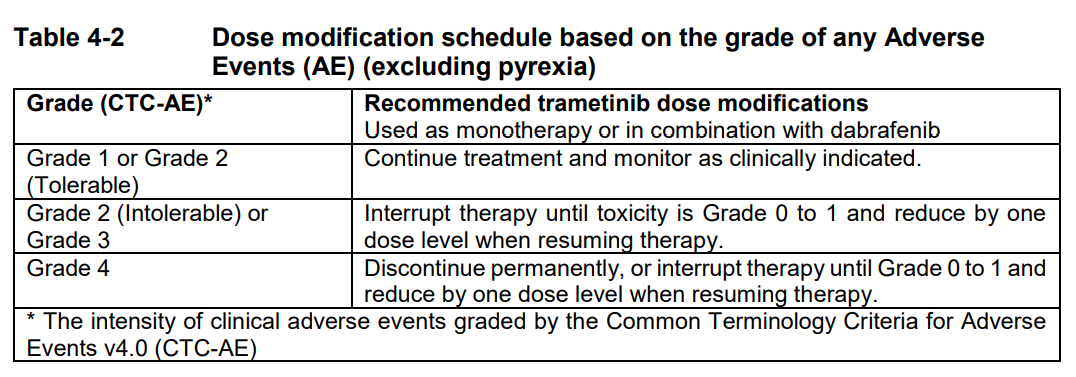 If treatment-related toxicities occur when trametinib is used in combination with dabrafenib, then both treatments should be simultaneously dose reduced, interrupted or discontinued. Exceptions where dose modifications are necessary for only one of the two treatments are detailed below for pyrexia, uveitis, RAS mutation positive non-cutaneous malignancies (primarily related to dabrafenib), left ventricular ejection fraction (LVEF) reduction, retinal vein occlusion (RVO), retinal pigment epithelial detachment (RPED) and interstitial lung disease (ILD)/pneumonitis (primarily related to trametinib). _Dose modification exceptions (where only one of the two therapies is dose reduced) for selected adverse reactions_ **Pyrexia** Therapy should be interrupted (Mekinist when used as monotherapy, and both Mekinist and Tafinlar when used in combination) if the patient’s temperature is ≥38°C (100.4°F). In case of recurrence, therapy can also be interrupted at the first symptom of pyrexia. Treatment with anti-pyretics such as ibuprofen or acetaminophen/paracetamol should be initiated. Patients should be evaluated for signs and symptoms of infection (see section 6 Warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Mekinist, or both Mekinist and Tafinlar when used in combination, should be restarted if patient is symptom free for at least 24 hours either (1) at the same dose level, or (2) reduced by one dose level, if pyrexia is recurrent and/or was accompanied by other severe symptoms including dehydration, hypotension, or renal failure. The use of oral corticosteroids should be considered in those instances in which anti-pyretics are insufficient. **Uveitis** No dose modifications are required for uveitis as long as effective local therapies can control ocular inflammation. If uveitis does not respond to local ocular therapy, dabrafenib should be withheld until resolution of ocular inflammation and then dabrafenib should be restarted reduced by one dose level. No dose modification of trametinib is required when taken in combination with dabrafenib _(see section Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. **RAS-mutation-positive non-cutaneous malignancies** Consider the benefits and risks before continuing treatment with dabrafenib in patients with a non-cutaneous malignancy that has a RAS mutation. No dose modification of trametinib is required when taken in combination with dabrafenib. **Left ventricular ejection fraction (LVEF) reduction/Left ventricular dysfunction** Trametinib should be interrupted in patients who have an asymptomatic, absolute decrease of >10% in LVEF compared to baseline and the ejection fraction below the institution’s lower limit of normal (LLN) _(see section Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. No dose modification of dabrafenib is required when trametinib is taken in combination with dabrafenib. If the LVEF recovers, treatment with trametinib may be restarted, but the dose should be reduced by one dose level with careful monitoring ( _see section Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Trametinib should be permanently discontinued in patients with Grade 3 or 4 left ventricular cardiac dysfunction or clinically significant LVEF reduction which does not recover within 4 weeks ( _see section Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Retinal vein occlusion (RVO) and Retinal pigment epithelial detachment (RPED)** If patients report new visual disturbances such as diminished central vision, blurred vision, or loss of vision at any time while on trametinib therapy, a prompt ophthalmological assessment is recommended. In patients who are diagnosed with RVO, treatment with trametinib, whether given as monotherapy or in combination with dabrafenib, should be permanently discontinued. No dose modification of dabrafenib is required when trametinib is taken in combination with dabrafenib. If RPED is diagnosed, follow the dose modification schedule in Table 4-3 below for trametinib ( _see section Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 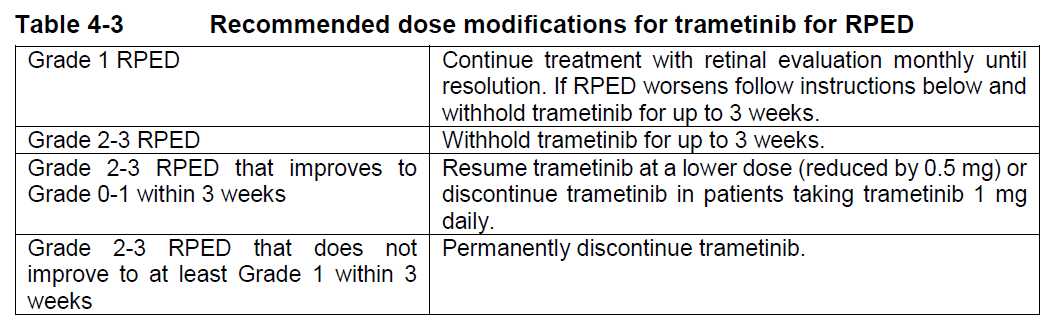 **Interstitial lung disease (ILD)/Pneumonitis** Withhold trametinib in patients with suspected ILD or pneumonitis, including patients presenting with new or progressive pulmonary symptoms and findings including cough, dyspnoea, hypoxia, pleural effusion, or infiltrates, pending clinical investigations. Permanently discontinue trametinib for patients diagnosed with treatment-related ILD or pneumonitis. No dose modification of dabrafenib is required when trametinib is taken in combination with dabrafenib for cases of ILD or pneumonitis. **Special populations** **Renal impairment** No dosage adjustment is required in patients with mild or moderate renal impairment. Mild or moderate renal impairment had no significant effect on the population pharmacokinetics of Mekinist ( _see section Clinical pharmacology, Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There are no clinical data in patients with severe renal impairment; therefore, the potential need for starting dose adjustment cannot be determined. Trametinib should be used with caution in patients with severe renal impairment when administered as monotherapy or in combination with dabrafenib. **Hepatic impairment** No dosage adjustment is required in patients with mild hepatic impairment. In a population pharmacokinetic analysis, trametinib oral clearance and thus exposure was not significantly different in patients with mild hepatic impairment compared to patients with normal hepatic function. Available data in patients with moderate or severe hepatic impairment from a clinical pharmacology study indicate a limited impact on trametinib exposure (see _section Clinical pharmacology, Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. Trametinib should be used with caution in patients with moderate or severe hepatic impairment when administered as monotherapy or in combination with dabrafenib. **Non-Caucasian patients** The safety and efficacy of trametinib in non-Caucasian patients have not been established. No data are available. **Geriatric patients (65 years of age or above)** No dose adjustment is required in patients 65 years of age or older _(see section Clinical pharmacology, Pharmacokinetics_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. More frequent dose adjustments (see Tables 4-1 and 4-2 above) may be required in patients 65 years of age or older ( _see section Adverse Drug Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Pediatric patients (below 18 years)** The safety and efficacy of trametinib in pediatric patients have not been established. Mekinist is not recommended in this age group. No data are available. Studies in juvenile animals have shown adverse effects of trametinib which had not been observed in adult animals ( _see section Non-clinical safety data_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of administration** Trametinib should be taken orally with a full glass of water. Trametinib tablets should not be chewed or crushed. Trametinib should be taken without food, at least 1 hour before or 2 hours after a meal _(see section Clinical pharmacology_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. It is recommended that the dose of trametinib is taken at a similar time every day. When trametinib and dabrafenib are taken in combination, the once-daily dose of trametinib should be taken at the same time each day with either the morning dose or the evening dose of dabrafenib. If a patient vomits after taking trametinib, the patient should not retake the dose and should take the next scheduled dose. Please refer to dabrafenib Package Insert for information on method of administration when given in combination with trametinib.
ORAL
Medical Information
**3 Indications** **Unresectable or metastatic melanoma** Trametinib as monotherapy or in combination with dabrafenib is indicated for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation ( _see section 6 Warnings and Precautions and section 12 Clinical studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Trametinib monotherapy has not demonstrated clinical activity in patients who have progressed on a prior BRAF inhibitor therapy _(see section 12 Clinical studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. **Adjuvant treatment of melanoma** Trametinib in combination with dabrafenib is indicated for the adjuvant treatment of patients with melanoma with BRAF V600 mutation and involvement of lymph node(s), following complete resection. **Advanced non-small cell lung cancer** Trametinib in combination with dabrafenib is indicated for the treatment of adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAF V600 mutation. **Locally advanced or metastatic anaplastic thyroid cancer** Trametinib in combination with dabrafenib is indicated for the treatment of patients with locally advanced or metastatic anaplastic thyroid cancer (ATC) with a BRAF V600 mutation and with no satisfactory locoregional treatment options _(see section 12 Clinical studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_.
**5 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed.
L01XE25
xl 01 xe 25
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
GlaxoSmithKline Manufacturing SpA
Glaxo Wellcome, S.A. (Primary and Secondary Packager)
Novartis Pharma Stein AG
Lek Pharmaceuticals d.d. (Primary and Secondary Packager)
Active Ingredients
Documents
Package Inserts
Mekinist PI.pdf
Approved: March 15, 2023
