Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE, LIQUID FILLED
**4.2 – Posology and method of administration** Navelbine® soft capsules must be given strictly by the oral route. Navelbine® soft capsules should be swallowed with water without chewing or sucking the capsule. It is recommended to take the capsule with some food. - **As a single agent:** The recommended regimen is: **First three administrations** 60 mg/m2 of body surface area, administered once weekly. **Subsequent administrations** Beyond the third administration, it is recommended to increase the dose of Navelbine® to 80 mg/m2 once weekly except in those patients for whom the neutrophil count dropped once below 500/mm3 or more than once between 500 and 1000/mm3 during the first three administrations at 60 mg/m2. 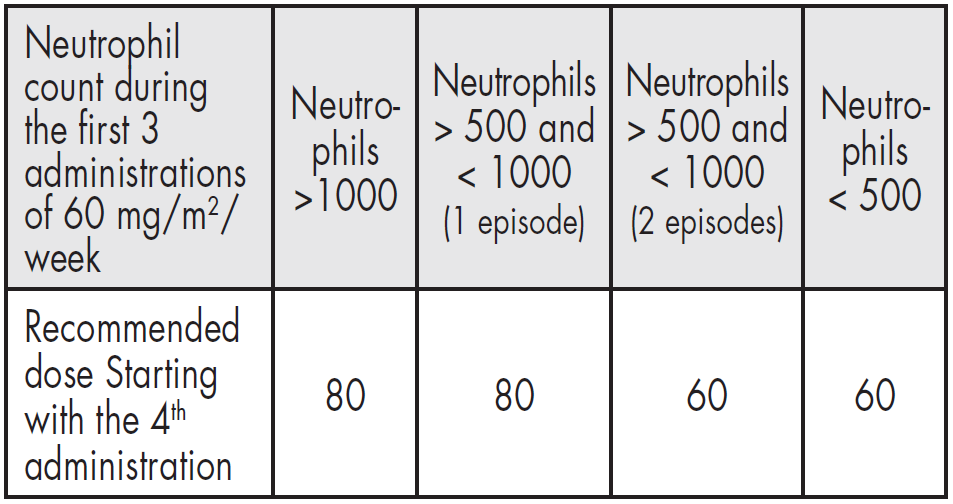 - **Dose Modification** For any administration planned to be given at 80 mg/m2, if the neutrophil count is below 500/mm3, the administration should be delayed until recovery and the dose reduced from 80 to 60 mg/m2 per week during the 3 following administrations. 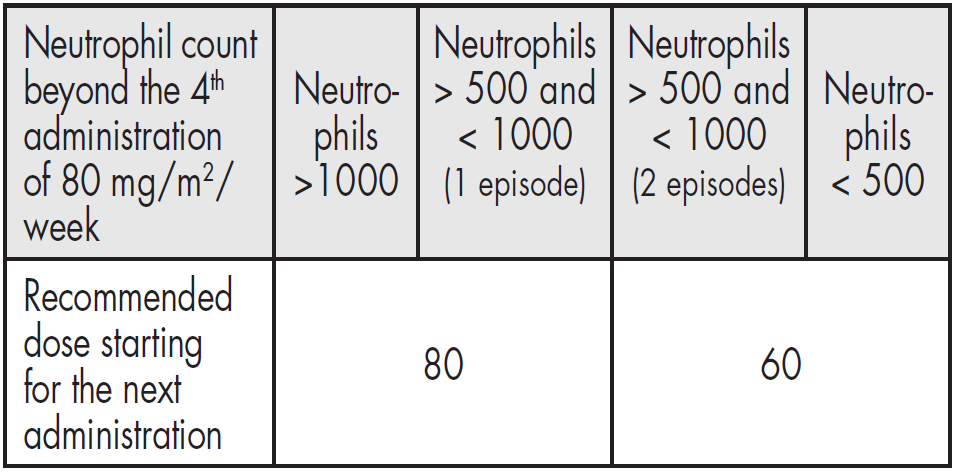 It is possible to re-escalate the dose from 60 to 80 mg/m2 per week if the neutrophil count did not drop below 500/mm3 or more than once between 500 and 1000/mm3 during 3 administrations given at 60 mg/m2 according to the rules previously defined for the first 3 administrations. - **For combination regimens, the dose and schedule will be adapted to the treatment protocol** Based on clinical studies, the oral dose of 80 mg/m2 was demonstrated to correspond to 30 mg/m2 of the IV form and 60 mg/m2 to 25 mg/m2. This has been the base for combination regimens alternating IV and oral forms improving patient’s convenience. For combination regimens, the dose and schedule will be adapted to the treatment protocol. The following table gives the dose required for appropriate ranges of body surface area (BSA). 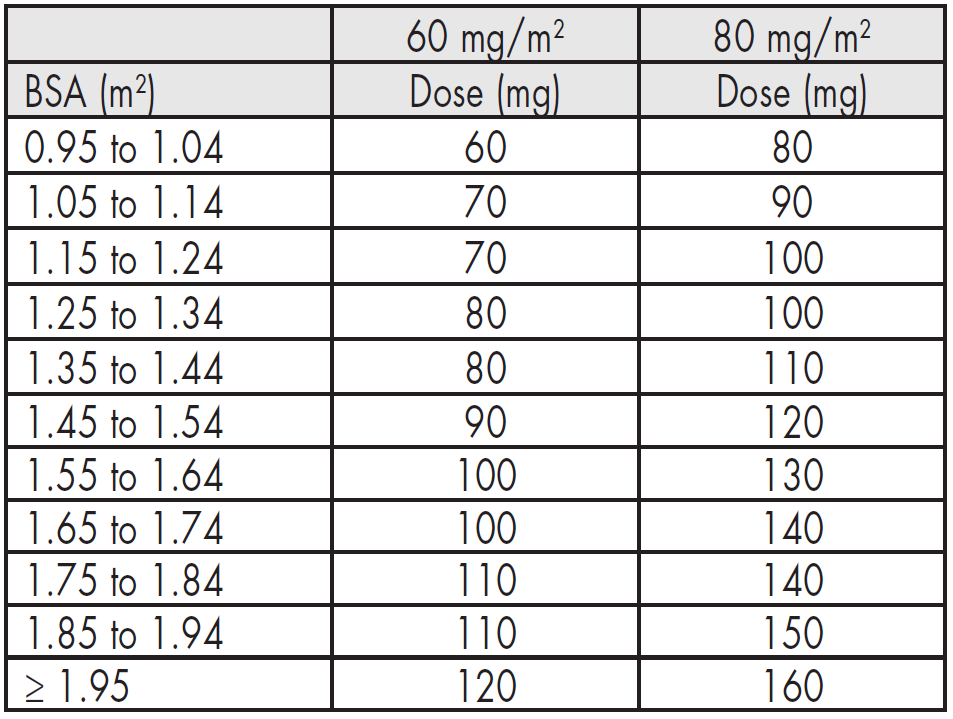 Even for patients with BSA ≥ 2 m2 the total dose should never exceed 120 mg per week at 60 mg/m2 and 160 mg per week at 80 mg/m2. **Administration in the elderly** Clinical experience has not detected any significant differences among elderly patients with regards to response rate, although greater sensitivity in some of these patients cannot be excluded. Age does not modify the pharmacokinetics of vinoreline (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Administration in children** Safety and efficacy in children have not been established and administration is therefore not recommended (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Administration in patients with liver insufficiency** Navelbine® can be administered at the standard dose of 60 mg/m2/week in patients with mild liver impairment (bilirubin < 1.5 x ULN, and ALAT and/or ASAT from 1.5 to 2.5 x ULN). In patients with moderate liver impairment (bilirubin from 1.5 to 3 x ULN, whatever the levels of ALAT and ASAT), Navelbine® should be administered at a dose of 50 mg/m2/week. The administration of Navelbine® in patients with severe hepatic impairment is not recommended as there is insufficient data to determine the pharmacokinetics, efficacy and safety of Navelbine® in this population (see sections 4.3, 4.4, 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Administration in patients with renal insufficiency** Given the minor renal excretion, there is no pharmacokinetic justification for reducing the dose of Navelbine® in patients with serious renal insufficiency (see sections 4.4, 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Instructions for use and / handling of oral Navelbine® (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**4.1 – Therapeutic indications** Non-small cell lung cancer – Advanced Breast Cancer
**4.3 – Contra-indications** - Known hypersensitivity to vinorelbine or other vinca alkaloids or to any of the constituent. - Disease significantly affecting absorption. - Previous significant surgical resection of stomach or small bowel. - Neutrophil count < 1500/mm3 or severe infection current or recent (within 2 weeks). - Platelet count < 100000/mm3. - Patients requiring long-term oxygen therapy. - Lactation (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - In combination with yellow fever vaccine (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01CA04
vinorelbine
Manufacturer Information
PIERRE FABRE SINGAPORE PTE. LTD.
CATALENT GERMANY EBERBACH GMBH
FAREVA PAU 1 (Primary/Secondary packager)
Active Ingredients
Documents
Package Inserts
Navelbine Capsule PI.pdf
Approved: November 16, 2022
