Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and Method of Administration** **Recommended Dose and Dosage Adjustment** _NINLARO® in combination with lenalidomide and dexamethasone_ The recommended starting dose of NINLARO® is 4 mg (one capsule) administered orally once a week on Days 1, 8, and 15 of a 28-day treatment cycle. The recommended starting dose of lenalidomide is 25 mg administered daily on Days 1 through 21 of a 28-day treatment cycle. The recommended starting dose of dexamethasone is 40 mg administered on Days 1, 8, 15, and 22 of a 28-day treatment cycle. 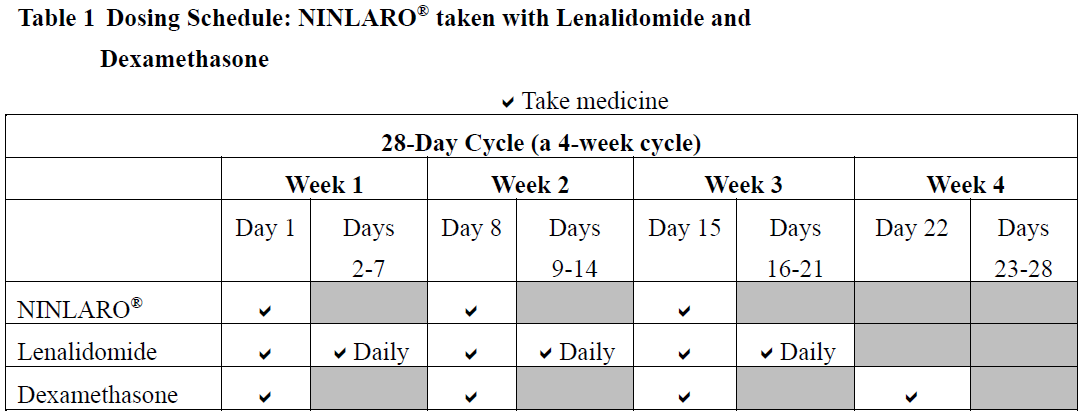 For additional information regarding lenalidomide and dexamethasone, refer to their respective product information. Prior to initiating a new cycle of therapy: - Absolute neutrophil count should be ≥ 1,000/mm3 - Platelet count should be ≥ 75,000/mm3 - Non-hematologic toxicities should, at the physician’s discretion, generally be recovered to patient’s baseline condition or ≤ Grade 1 Treatment should be continued until disease progression or unacceptable toxicity. _**Delayed or Missed Doses**_ In the event that a NINLARO® dose is delayed or missed, the dose should be taken only if the next scheduled dose is ≥ 72 hours away. A missed dose should not be taken within 72 hours of the next scheduled dose. A double dose should not be taken to make up for a missed dose. If a patient vomits after taking a dose, the patient should not repeat the dose but should resume dosing at the time of the next scheduled dose. _**Dose Modifications**_ The NINLARO® dose reduction steps in combination with lenalidomide and dexamethasone are presented in Table 2 and the dose modification guidelines are provided in Table 3. 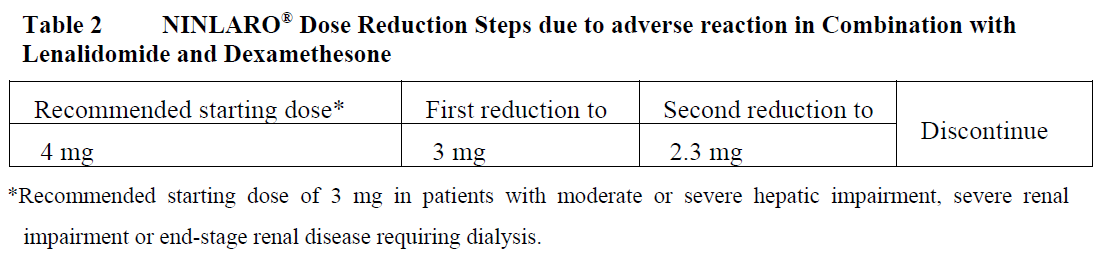 An alternating dose modification approach is recommended for NINLARO® and lenalidomide for overlapping toxicities of thrombocytopenia, neutropenia, and rash as described in Table 3. Refer to the lenalidomide product information for dose modification guidelines if dose modification is needed for lenalidomide. 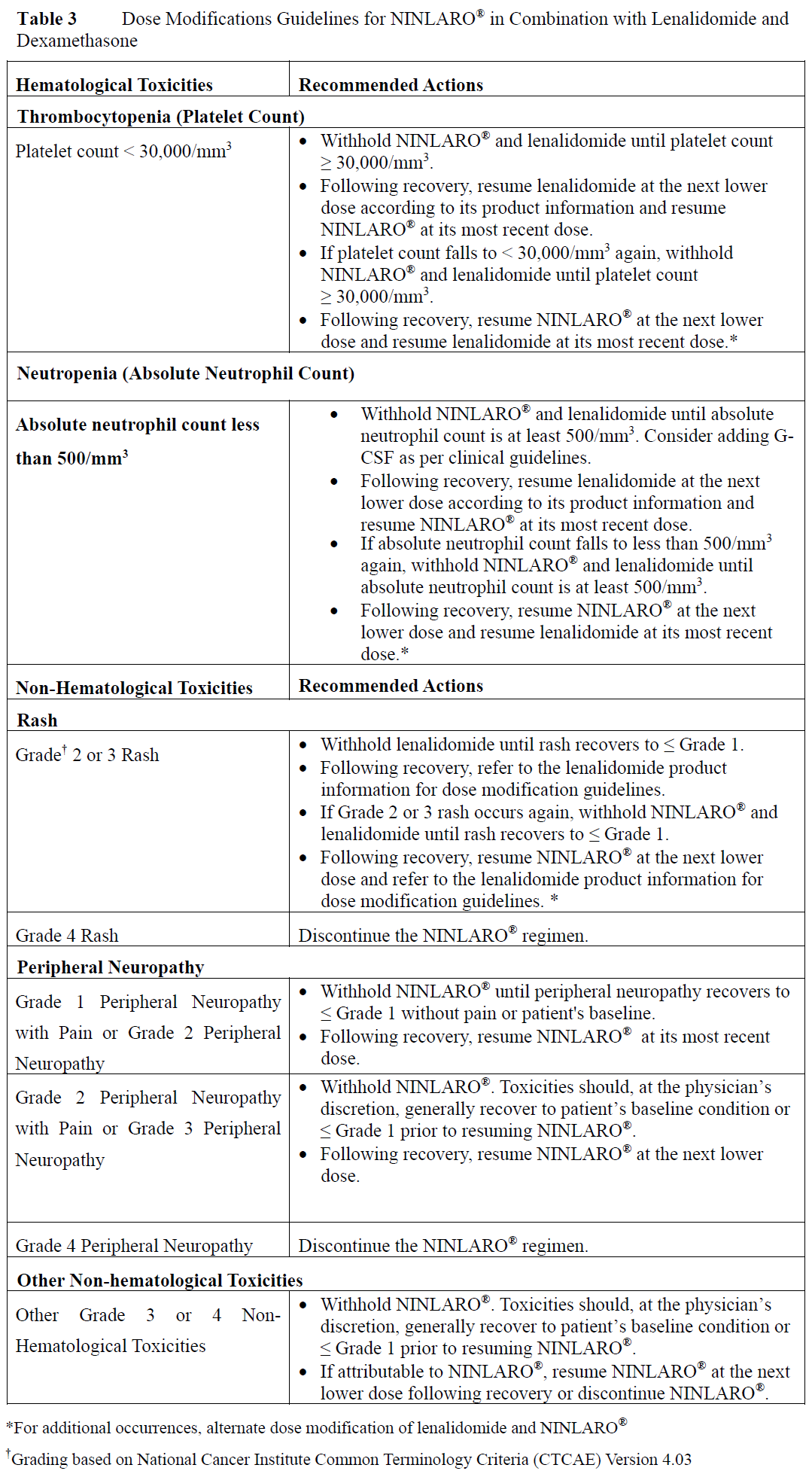 _**Concomitant Medicinal Products**_ Antiviral prophylaxis should be considered in patients being treated with NINLARO® to decrease the risk of herpes zoster reactivation. Patients treated in the NINLARO® regimen who received antiviral prophylaxis had a lower incidence (< 1%) of herpes zoster infection compared to patients who did not receive prophylaxis (6%). _**Special Patient Populations**_ **Geriatrics (≥ 65 years of age):** No dose adjustment of NINLARO® is required for patients over 65 years of age based on the results of a population PK analysis. In studies of NINLARO®, there were no clinically significant differences in safety and efficacy between patients less than 65 years of age and patients 65 years of age or older. **Pediatric Patients (< 18 years of age)** The safety and efficacy of NINLARO® in children below 18 years of age have not been established. **Hepatic Impairment** No dose adjustment of NINLARO® is required for patients with mild hepatic impairment (total bilirubin ≤ upper limit of normal (ULN) and aspartate aminotransferase (AST) > ULN or total bilirubin > 1–1.5 x ULN and any AST) based on the results of a population pharmacokinetic (PK) analysis. A lower starting dose of 3 mg is recommended for patients with moderate (total bilirubin > 1.5–3 x ULN) or severe (total bilirubin > 3 x ULN) hepatic impairment based on the results of a PK study (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal Impairment** No dose adjustment of NINLARO® is required for patients with mild or moderate renal impairment (creatinine clearance ≥ 30 mL/min) based on the results of a population PK analysis. A lower starting dose of 3 mg is recommended for patients with severe renal impairment (creatinine clearance < 30 mL/min) or end-stage renal disease (ESRD) requiring dialysis based on the results of a PK study. NINLARO® is not dialyzable and therefore can be administered without regard to the timing of dialysis (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Refer to the lenalidomide product information for dosing recommendations in patients with renal impairment. **Method of Administration** NINLARO® should be taken once a week on the same day and at approximately the same time for the first three weeks of a four week cycle. NINLARO® should be taken at least one hour before or at least two hours after food (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The capsule should be swallowed whole with water. The capsule should not be crushed, chewed or opened. Direct contact with capsule contents should be avoided as NINLARO® may be harmful by inhalation, ingestion, or skin absorption (see Section 6.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**4.1 Therapeutic Indications** NINLARO® \[ixazomib (as ixazomib citrate)\] in combination with lenalidomide and dexamethasone is indicated for the treatment of adult patients with multiple myeloma who have received at least one prior therapy.
**4.3 Contraindications** Patients who are hypersensitive to this drug or to any ingredient in the formulation or component of the container. For a complete listing, refer to Section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01XX50
xl 01 xx 50
Manufacturer Information
TAKEDA PHARMACEUTICALS (ASIA PACIFIC) PTE. LTD.
HAUPT PHARMA AMAREG GMBH
ANDERSONBRECON (UK) LIMITED (TALGARTH) (primary and secondary packager)
Takeda Ireland Ltd.
Active Ingredients
Documents
Package Inserts
Ninlaro Capsule PI.pdf
Approved: August 3, 2022
