Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**DOSAGE AND ADMINISTRATION** **For Intravenous Use Only** **Dose** Primary Immunodeficiency (PI) The recommended dose of GAMMAGARD S/D for patients with PI is 300–600 mg/kg infused at 3 to 4 week intervals are commonly used.5,6,12 Adjust dose according to the clinical response; the frequency and amount of immunoglobulin therapy may vary from patient to patient. No randomized controlled clinical trials are available to determine an optimum target trough serum IgG level. B-cell Chronic Lymphocytic Leukemia (CLL) The recommended dose of GAMMAGARD S/D for patients with hypogammaglobulinemia and/or recurrent bacterial infections due to B-cell CLL is 400 mg/kg body weight infused at every 3 to 4 week intervals.3 Kawasaki Syndrome The recommended dose of GAMMAGARD S/D for patients with Kawasaki syndrome is either a single 1 g/kg dose or a dose of 400 mg/kg for four consecutive days beginning within seven days of the onset of fever, administered concomitantly with appropriate aspirin therapy (80–100 mg/kg/day in four divided doses).4,8,13 Idiopathic Thrombocytopenic Purpura (ITP) The recommended dose of GAMMAGARD S/D for patients with acute or chronic ITP is 1 g/kg. The need for additional doses can be determined by clinical response and platelet count. Up to three separate doses may be given on alternate days if required. **Preparation and Handling** Allow GAMMAGARD S/D and diluent to reach room temperature before reconstitution and administration if refrigerated. **Reconstitution:** 1\. Remove caps from concentrate and diluent bottles to expose central portion of stoppers and cleanse stopper with germicidal solution. To make a **5% solution:** Use the full volume of the diluent bottle To make a **10% solution:** Remove half of the volume of the diluent bottle Table 7 indicates the volume of diluent that should be removed from the vial before attaching the transfer device to produce a 10% concentration. Using aseptic technique, withdraw the unnecessary volume of diluent using a sterile hypodermic syringe and needle. Discard the filled syringe into a suitable puncture proof container (Sharps container).  2\. Remove spike cap from one end of the transfer device. Do not touch spike. 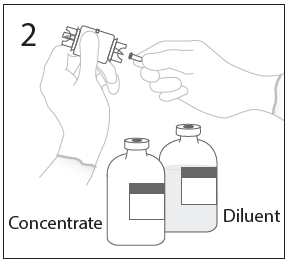 3a. Place diluent bottle on a flat surface. Use exposed end of transfer device to spike diluent bottle through center of the stopper. CAUTION: Failure to insert spike into center of the stopper may result in dislodging of the stopper. 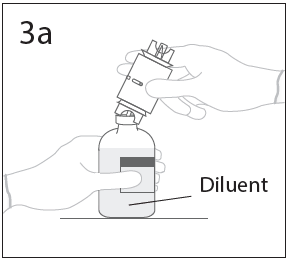 3b. Ensure that the collar collapses fully into the device by pushing down on the transfer device firmly. While holding onto transfer device, remove remaining spike cover. Do not touch spike. 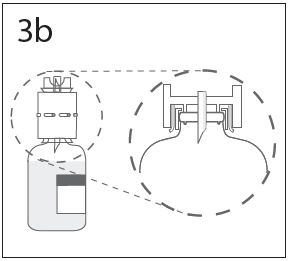 4\. Hold diluent bottle with attached transfer device at an angle to the concentrate bottle to prevent spilling the diluent. Note: Do not hold diluent bottle upside down, for this can lead to diluent spillage. 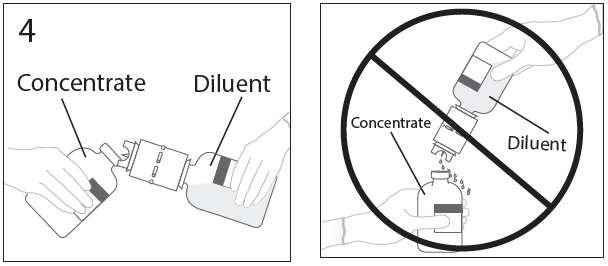 5a. Spike concentrate bottle through center of the stopper while **quickly inverting the diluent vial** to minimize spilling out diluent. CAUTION: Failure to insert spike into the center of the stopper may result in dislodging of the stopper and loss of vacuum. 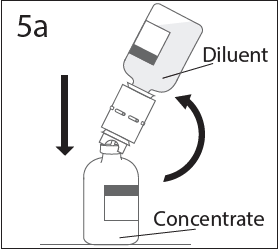 5b. Ensure that the collar collapses fully into the device by pushing down on the diluent bottle firmly. 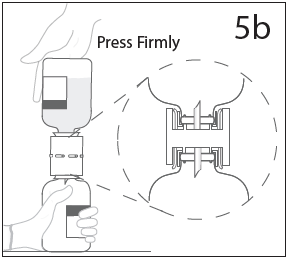 6\. After transfer of diluent is complete, remove transfer device and empty diluent bottle. Immediately swirl concentrate bottle gently to thoroughly mix contents. CAUTION: Do not shake. Avoid foaming. Discard transfer device after single use per local guidelines. 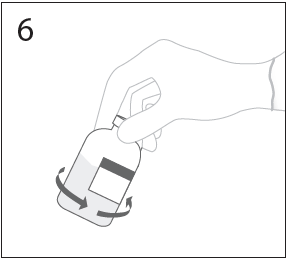 Visually inspect parenteral drug product for particulate matter and discoloration prior to administration. Reconstituted GAMMAGARD S/D is a clear to slightly opalescent and colorless to pale yellow solution. Do not use if particulate matter and/or discoloration is observed. Follow directions for use which accompany the administration set provided. If another administration set is used, ensure that the set contains a similar filter. Begin administration as soon as possible within 2 hours if reconstitution is performed aseptically outside of a sterile laminar air flow hood. Administer within 24 hours if reconstitution is performed aseptically inside of a sterile laminar flow hood and stored in the original glass container or pooled into ViaFlex bags under constant refrigeration (2 °C to 8 °C). Record the date and time of reconstitution/pooling. Discard partially used vials. **Administration** GAMMAGARD S/D, Immune Globulin Intravenous (Human), should only be administered intravenously. Other routes of administration have not been evaluated. Administer GAMMAGARD S/D as soon after reconstitution as possible and administer the reconstituted material at room temperature. It is recommended that initially a 5% solution be infused at a rate of 0.5 mL/kg/Hr. Infusion rate may be gradually increased to a maximum rate of 4 mL/kg/Hr as tolerated for patients with no history of adverse reactions to IGIV and no significant risk factors for renal dysfunction or thrombotic complications. Patients who tolerate the 5% concentration at 4 mL/kg/Hr can be infused with the 10% concentration starting at 0.5 mL/kg/Hr. The rate can be increased gradually up to a maximum of 8 mL/kg/Hr if no adverse effects occur14. Monitor patient vital signs throughout the infusion. Certain adverse reactions such as headaches, flushing and changes in pulse rate and blood pressure may be related to the rate of infusion. Slow or stop the infusion if adverse reactions occur. If symptoms subside promptly, the infusion may be resumed at a lower rate that does not result in reoccurrence of the symptoms. It is recommended that, if possible, the antecubital veins are used, especially for 10% solutions, to reduce the likelihood of discomfort at the infusion site (see **ADVERSE REACTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Adverse reactions may occur more frequently in patients especially those with immune deficiency who receive human IGIV for the first time, upon switching brands, or if there has been a long hiatus since the previous infusion. In such cases, start at a lower rate and gradually increase as tolerated. There are no prospective studies demonstrating that any concentration or rate of infusion is completely safe. However, the risk may be decreased at lower rates of infusion.8 For patients who are judged to be at risk of renal dysfunction or thrombotic complications, administer GAMMAGARD S/D at a minimum allowable rate of infusion and gradually titrated up to a more conservative maximal rate of less than 3.3 mg/kg/min (< 2 mL/kg/hr of a 10% or < 4 mL/kg/hr of a 5% solution) (see **WARNINGS AND PRECAUTIONS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). * * * **REFERENCES** 3 Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia: Intravenous immunoglobulin for the prevention of infection in Chronic Lymphocytic Leukemia: A randomized, controlled clinical trial. N Eng J Med. 1988; 319:902–907. 4 Barron KS, Murphy DJ, Siverman ED, Ruttenberg HD, Wright GB, Franklin W, Goldberg SJ, Higashino SM, Cox DG, Lee M. Treatment of Kawasaki syndrome: a comparison of two dosage regimens of intravenously administered immune globulin. J Pediatr. 1990; 117:638–644. 5 Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, Buckley R, Chinen J, El-Gamal Y, Mazer BD, Nelson Jr. RP, Patel DD, Secord E, Sorenson RU, Wasserman RL, Cunningham-Rundles C. Use of Intravenous Immunoglobulin in Human Disease: A Review of Evidence by Members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol 2006; 117:S525–53. 6 Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005; 94(suppl 1):S1–63. 8 Newburger J, Takahashi M, Burns JG, et al. The Treatment of Kawasaki Syndrome with Intravenous Gamma Globulin. New England Journal of Medicine. 1986; 315:341–347. 12 Eijkhout HW, Der Meer JW, Kallenbert CG, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann Intern Med. 2001; 135:165–174. 13 Engle MA, Fatica NS, Bussel JB, O’Laughlin JE, Snyder MS, Lesser ML. Clinical Trial of Single-Dose Intravenous Gammaglobulin in Acute Kawasaki Disease. AJDC. 1989; 143:1300–1304. 14 Polmar SH, Smith TF, Pirofsky B, Cox DG, Rechtman D. Rapid infusion of intravenous immunoglobulin in patients with primary immunodeficiency disease. J Allergy Clin Immunol. 1992; 69:166.
INTRAVENOUS
Medical Information
**INDICATIONS AND USAGE** GAMMAGARD S/D is not indicated in patients with selective IgA deficiency where the IgA deficiency is the only abnormality of concern (see **WARNINGS** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Primary Immunodeficiency (PI)** GAMMAGARD S/D is indicated for the treatment of primary immunodeficient states, such as: congenital agammaglobulinemia, common variable immunodeficiency, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.7,15 This indication was supported by a clinical trial of 17 patients with primary immunodeficiency who received a total of 341 infusions. GAMMAGARD S/D is especially useful when high levels or rapid elevation of circulating IgG are desired or when intramuscular injections are contraindicated (e.g., small muscle mass). **B-cell Chronic Lymphocytic Leukemia (CLL)** GAMMAGARD S/D is indicated for prevention of bacterial infections in patients with hypogammaglobulinemia and/or recurrent bacterial infections associated with B-cell chronic lymphocytic leukemia (CLL).3 **Idiopathic Thrombocytopenic Purpura (ITP)** When a rapid rise in platelet count is needed to prevent and/or to control bleeding in a patient with idiopathic thrombocytopenia purpura, the administration of GAMMAGARD S/D, should be considered. **Kawasaki Syndrome** GAMMAGARD S/D, is indicated for the prevention of coronary artery aneurysms associated with Kawasaki syndrome. 8 * * * **REFERENCES** 3 Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia: Intravenous immunoglobulin for the prevention of infection in Chronic Lymphocytic Leukemia: A randomized, controlled clinical trial. N Eng J Med. 1988; 319:902–907. 7 Buckley RH. Immunoglobulin replacement therapy: Indications and contraindications for use and variable IgG levels achieved In: Alving BM, Finlayson JS eds. Immunoglobulins: characteristics and use of intravenous preparations. Washington, D.C.: US Department of Health and Human Services; 1979;3–8. 8 Newburger J, Takahashi M, Burns JG, et al. The Treatment of Kawasaki Syndrome with Intravenous Gamma Globulin. New England Journal of Medicine. 1986; 315:341–347. 15 Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, Wedgwood RJ. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med. 1988; 112:634–40.
**CONTRAINDICATIONS** GAMMAGARD S/D is contraindicated in patients who have a history of anaphylactic or severe systemic hypersensitivity reactions to the administration of human immunoglobulin. GAMMAGARD S/D is contraindicated in IgA deficient patients with antibodies to IgA and a history of hypersensitivity.
J06BA02
immunoglobulins, normal human, for intravascular adm.
Manufacturer Information
TAKEDA PHARMACEUTICALS (ASIA PACIFIC) PTE. LTD.
Baxalta US Inc. (Intermediate)
Baxalta Belgium Manufacturing S.A.
Baxter Healthcare Corporation (Diluent)
Active Ingredients
Documents
Package Inserts
1.4.3 Gammagard SD PI_Proposed.pdf
Approved: May 8, 2020
