Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION, CONCENTRATE
**Posology and method of administration** IV: Unless prescribed otherwise, MESNA Stada is normally administered intravenously to adults at a dose of 20% of the oxazaphosphorine dose at time zero (the time of administration of the oxazaphosphorine), and then at 4 and 8 hours. 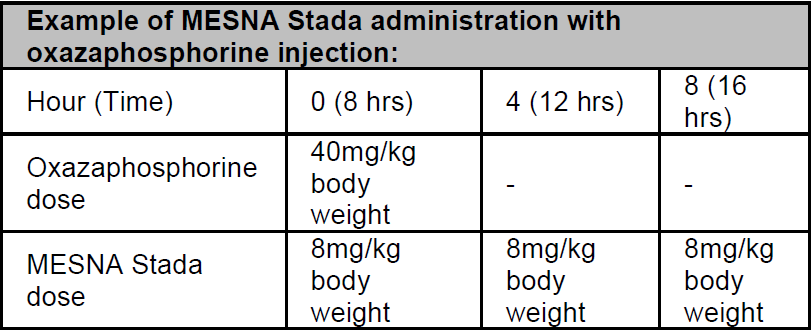 Clinical experience with children has shown that it is beneficial in individual cases to give MESNA Stada at shorter intervals (e.g., every 3 hrs, total MESNA Stada dose = 60% of oxazaphosphorine dose). With very high-dose oxazaphosphorine cytostatic therapy (e.g., before bone marrow transplantation), the total MESNA Stada dose can be increased to between 120% and 160% of the oxazaphosphorine dose. It is recommended that after administration of 20% MESNA Stada (related to the total dose of oxazaphosphorine) at time 0, the remaining calculated dose should be given continuously IV over a period of 24 hrs with a perfusor. Alternatively, an intermittent bolus injection is possible: For adults 3 x 40% (at times 0, 4, 8 hrs) or 4 x 40% (at times 0, 3, 6, 9 hrs), respectively. For children due to more frequent micturition, the bolus injections should always be given in 3-hr intervals (e.g., 20% at times 0, 1, 3, 6, 9, 12 hrs). Instead of a bolus injection, short infusions of 15-min duration are possible. With a continuous infusion of ifosfamide (Holoxan), it has been shown to be of benefit to give MESNA Stada at time zero following the initial 20% bolus injection (start of infusion, time 0), followed by infusion to up to 100% of the ifosfamide dose, and to continue uroprotection for a further 6 to 12 hours after termination of the ifosfamide infusion. 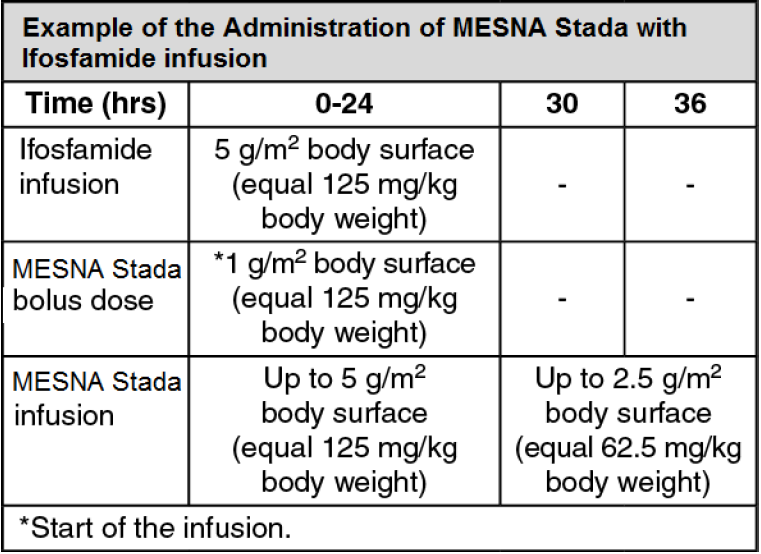
INTRAVENOUS
Medical Information
**Therapeutic indications** Prevention of urinary tract toxicity of oxazaphosphorines (e.g., ifosfamide, cyclophosphamide or trofosfamide). In the case of a tumour therapy with ifosfamide MESNA Stada must always be given. In the case of a tumour therapy with cyclophosphamide or trofosfamide, MESNA Stada must always be given when dosages above 10 mg/kg are used and in patients at risk. Risks are in particular: previous radiation therapy in the area of the true pelvis, cystitis following previous therapy with ifosfamide, cyclophosphamide or trofosfamide and a history of uropathy.
**Contraindications** Known hypersensitivity to mesna, other thiol compounds or to any of the excipients. As mesna is only indicated in combination with oxazaphosphorines, the contraindications which apply to cyclophosphamide, ifosfamide and trofosfamide should also be observed.
V03AF01
mesna
Manufacturer Information
DCH AURIGA SINGAPORE
Haupt Pharma Wolfratshausen GmbH
Active Ingredients
Documents
Package Inserts
MESNA STADA PI.pdf
Approved: November 30, 2022
