Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**4.2 Dose and method of administration** Dose Fosaprepitant-AFT, for administration by intravenous infusion, is a lyophilised prodrug of aprepitant containing polysorbate 80. Fosaprepitant-AFT 150 mg is administered on day 1 as an infusion over 20–30 minutes initiated approximately 30 minutes prior to chemotherapy. Fosaprepitant-AFT should be administered in conjunction with a corticosteroid and a 5-HT3 antagonist as specified in Tables 1 and Table 2. The package insert for the co-administered 5-HT3 antagonist must be consulted prior to initiation of treatment with Fosaprepitant-AFT 150 mg. 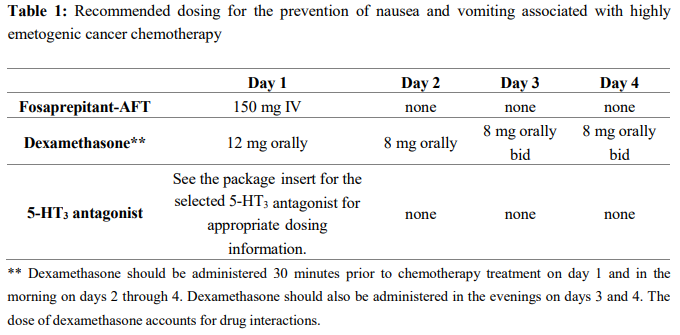 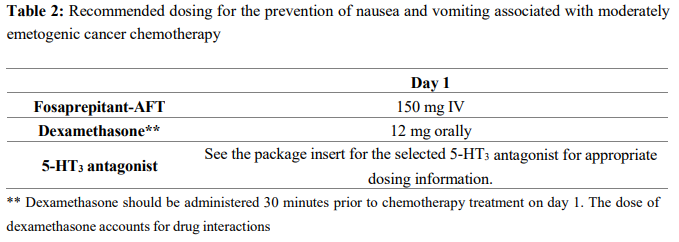 _**Special populations**_ No dosage adjustment is necessary based on age, gender, race, or Body Mass Index. _Renal impairment_ No dosage adjustment is necessary for patients with severe renal insufficiency (creatinine clearance <30 mL/min) or for patients with end stage renal disease undergoing haemodialysis. _Hepatic impairment_ No dosage adjustment is necessary for patients with mild to moderate hepatic insufficiency (Child-Pugh score 5 to 9). There are no clinical data in patients with severe hepatic insufficiency (Child-Pugh score >9). Method of administration 1. Inject 5 mL saline into the vial. Assure that saline is added to the vial along the vial wall in order to prevent foaming. Swirl the vial gently. Avoid shaking and jetting saline into the vial. 2. Aseptically prepare an infusion bag filled with 145 mL of saline. 3. Aseptically withdraw the entire volume from the vial and transfer it into an infusion bag containing 145 mL of saline to yield a total volume of 150 mL. Gently invert the bag 2–3 times.\* 4. To avoid microbiological hazard, Fosaprepitant-AFT solution should be used as soon as practicable after reconstitution and further dilution. If storage is unavoidable, the solution should be held below 25°C for not more than 24 hours. 5. Parenteral drug products should be inspected visually for particulate matter and discolouration before administration whenever solution and container permit. 6. Fosaprepitant-AFT 150 mg should only be administered as an infusion over **20–30 minutes**. Fosaprepitant-AFT is for single use in one patient only. Discard any residue. \\* Please Note: there is a 5% overfill in each vial to account for non-withdrawable losses and to ensure that the labelled dose of 150 mg is deliverable after reconstitution. General information See Section 4.5 Interactions with Other Medicines and Other Forms of Interactions for additional information on the administration of Fosaprepitant-AFT with corticosteroids – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Refer to the full prescribing information for coadministered antiemetic agents.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Fosaprepitant-AFT, in combination with a corticosteroid and a 5-HT3 antagonist, is indicated for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of: - highly emetogenic cancer chemotherapy (see Section 4.2 Dose and Method of Administration) - moderately emetogenic cancer chemotherapy (see Section 4.2 Dose and Method of Administration).
**4.3 Contraindications** Fosaprepitant-AFT is contraindicated in patients who are hypersensitive to Fosaprepitant-AFT, aprepitant, polysorbate 80 or any other components of the product. Fosaprepitant-AFT should not be used concurrently with pimozide, terfenadine, astemizole, or cisapride. Inhibition of cytochrome P450 isoenzyme 3A4 (CYP3A4) by aprepitant could result in elevated plasma concentrations of these drugs, potentially causing serious or life-threatening reactions (see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
A04AD12
aprepitant
Manufacturer Information
APEX PHARMA MARKETING PTE. LTD.
QILU PHARMACEUTICAL (HAINAN) CO., LTD.
Active Ingredients
Documents
Package Inserts
FOSAPREPITANT-AFT POWDER FOR INJECTION PI.pdf
Approved: January 24, 2022
