Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**2 DOSAGE AND ADMINISTRATION** **2.1 Recommended Dosage** The recommended dosage of AKYNZEO IV and dexamethasone in adults for the prevention of nausea and vomiting associated with administration of emetogenic chemotherapy are shown in Table 1. 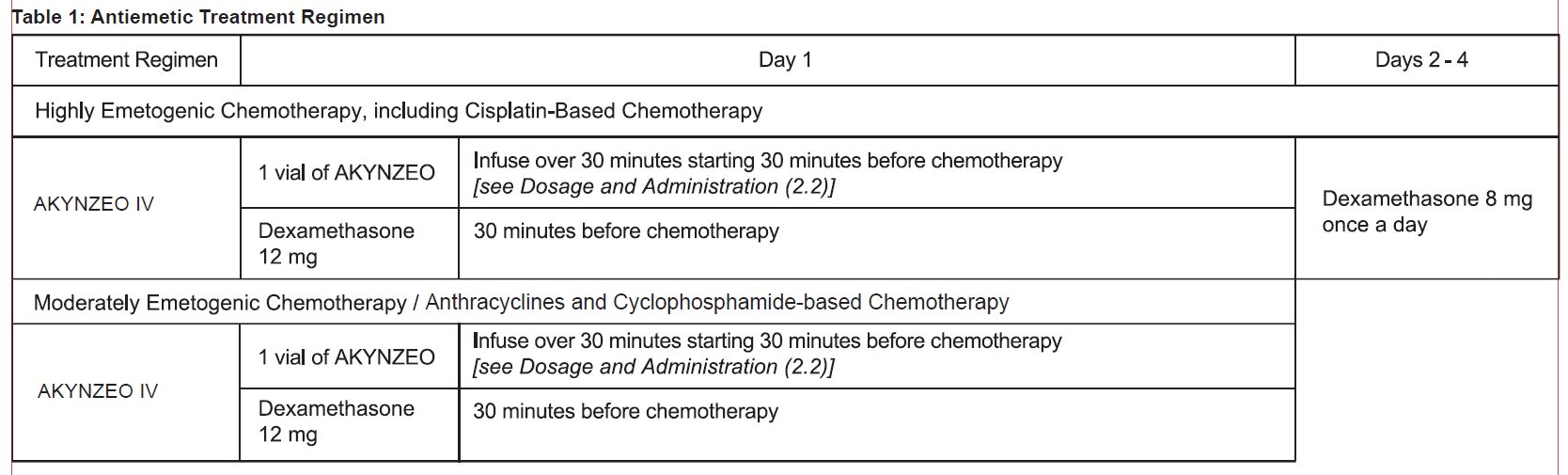 **2.2 Preparation and Administration of AKYNZEO IV** AKYNZEO IV is for use as an intravenous infusion after dilution. AKYNZEO IV contains no antimicrobial preservatives, is intended for single use only and is compatible with intravenous dexamethasone sodium phosphate which can be added to the AKYNZEO IV solution or infused simultaneously. See Table 2 for preparation instructions of AKYNZEO IV for intravenous infusion. 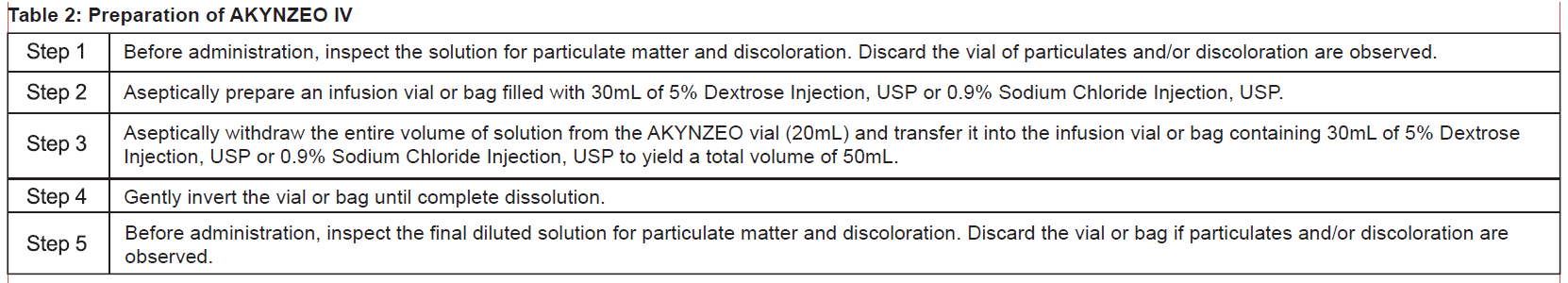 Storage The total time from dilution to the start of the infusion, with or without intravenous dexamethasone sodium phosphate, should not exceed 24 hours. Store the final diluted solution at below 25°C. Administration Administer over 30 minutes as an intravenous infusion. At the end of the infusion, flush the infusion line with the same carrier solution to ensure complete drug administration. **2.3 Incompatibility of AKYNZEO IV** AKYNZEO IV is incompatible with any solution containing divalent cations (e.g., calcium, magnesium), including Lactated Ringer’s Injection and Hartmann’s Solution. Limited data are available on the compatibility of AKYNZEO IV with other intravenous substances, additives or other medications with the exception of intravenous dexamethasone sodium phosphate \[see _Dosage and Administration (2.2)_\], and they should not be added to the AKYNZEO solution or infused simultaneously. If the same intravenous line is used for sequential infusion of several different drugs, flush the line before and after infusion of AKYNZEO solution with 0.9% Sodium Chloride Injection, USP.
INTRAVENOUS
Medical Information
**1 INDICATIONS AND USAGE** AKYNZEO IV is indicated in adults for the: - Prevention of acute and delayed nausea and vomiting associated with highly emetogenic chemotherapy, including cisplatin. - Prevention of acute and delayed nausea and vomiting associated with moderately emetogenic cancer chemotherapy.
**4 CONTRAINDICATIONS** Pregnancy
A04AA55
palonosetron, combinations
Manufacturer Information
Juniper Biologics Pte Ltd.
Baxter Oncology GmbH
Active Ingredients
Documents
Package Inserts
Akynzeo IV Concentrate for Solution for Infusion PI.pdf
Approved: November 28, 2023
