Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
OTHER
**POSOLOGY AND METHOD OF ADMINISTRATION** CERVIDIL® should only be administered by qualified healthcare personnel in hospitals and clinics with obstetric units with facilities for continuous fetal and uterine monitoring. After insertion, uterine activity and fetal condition must be carefully and regularly monitored. Posology One vaginal delivery system is administered high into the posterior vaginal fornix. 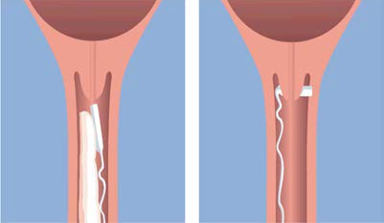 The vaginal delivery system should be removed after 24 hours irrespective of whether cervical ripening has been achieved. In case of subsequent administration of uterotonic drugs, a dosing interval of at least 30 minutes is recommended following the removal of the vaginal delivery system. _Paediatric population_ The safety and efficacy of CERVIDIL® in pregnant woman aged less than 18 years has not been established. No data are available. Method of administration _Administration_ CERVIDIL® should be removed from the freezer just prior to the insertion. No thawing is required prior to use. There is a “tear mark” on side of the foil sachet. Open the package along the tear mark across the top of the sachet. Do not use scissors or other sharp objects which may cut the retrieval system. The vaginal delivery system should be inserted high into the posterior vaginal fornix using only small amounts of water soluble lubricants to aid insertion. After the vaginal delivery system has been inserted, the withdrawal tape may be cut with scissors. Always ensuring there is sufficient tape outside the vagina to allow removal. No attempt should be made to tuck the end of the tape into the vagina as this may make retrieval more difficult. The patient should be recumbent for 30 minutes after insertion. As dinoprostone will be released continuously over a period of 24 hours, it is important to monitor uterine contractions and fetal condition at frequent regular intervals. _Removal_ The vaginal delivery system can be removed quickly and easily by gentle traction on the retrieval tape. It is necessary to remove the vaginal delivery system to terminate drug administration when cervical ripening is judged to be complete or for any of the reasons listed below. 1. Onset of labour. For the purposes of induction of labour with CERVIDIL®, the onset of labour is defined as the presence of regular painful uterine contractions occurring every 3 minutes irrespective of any cervical change. There are two important points to note: 1. Once regular, painful contractions have been established with CERVIDIL® they will not reduce in frequency or intensity as long as CERVIDIL® remains in situ because dinoprostone is still being administered. 2. Patients, particularly multigravidae, may develop regular painful contractions without any apparent cervical change. Effacement and dilatation of the cervix may not occur until uterine activity is established. Because of this, once regular painful uterine activity is established with CERVIDIL® in-situ, the vaginal delivery system should be removed irrespective of cervical state to avoid the risk of uterine hyperstimulation. 2. Spontaneous rupture of the membranes or amniotomy. 3. Any suggestion of uterine hyperstimulation or hypertonic uterine contractions. 4. Evidence of fetal distress. 5. Evidence of maternal systemic adverse dinoprostone effects such as nausea, vomiting, hypotension or tachycardia. 6. At least 30 minutes prior to starting an intravenous infusion of uterotonic drugs. The opening on one side of the retrieval device is present only to allow the manufacturer to enclose the vaginal delivery system into the retrieval device during manufacture. The vaginal delivery system should NEVER be removed from the retrieval device. Upon removal of the product from the vagina, the vaginal delivery system will have swollen to 2–3 times its original size and be pliable.
VAGINAL
Medical Information
**THERAPEUTIC INDICATIONS** Initiation of cervical ripening in patients, at or near term, who have favourable induction features and in whom there is a medical or obstetrical indication for induction of labour.
**CONTRAINDICATIONS** CERVIDIL® should not be used or left in place: 1. When labour has started. 2. When uterotonic drugs and/or other labour induction agents are being given. 3. When strong prolonged uterine contractions would be inappropriate such as in patients: 1. who have had previous major uterine surgery, e.g. caesarean section, myomectomy (see sections special warnings and precautions for use & undesirable effects – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) 2. who have had previous major uterine cervix surgery (e.g. other than biopsies and cervical abrasion) or rupture of the uterine cervix 3. with cephalopelvic disproportion 4. with fetal malpresentation 5. with suspicion or evidence of fetal distress 4. When there is current pelvic inflammatory disease, unless adequate prior treatment has been instituted. 5. When there is hypersensitivity to dinoprostone or to any of the excipients. 6. When there is placenta previa or active herpes genitalis or unexplained vaginal bleeding during the current pregnancy. 7. When the patient is carrying more than one fetus or the fetus is in a non-vertex presentation. 8. When there is abnormal cardiotocography or suspected fetal compromise. 9. In the presence of any suggestion of uterine hyperstimulation or hypertonic uterine contractions.
G02AD02
dinoprostone
Manufacturer Information
FERRING PHARMACEUTICALS PRIVATE LIMITED
Ferring Controlled Therapeutics Limited
Active Ingredients
Documents
Package Inserts
Cervidil Vaginal Delivery System 10mg PI.pdf
Approved: January 12, 2022
