Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**4.2 Posology and method of administration** Treatment should be initiated under the supervision of a physician experienced in the treatment of haemophilia. **Posology** The dosage and duration of the substitution therapy depend on the severity of the factor VIII deficiency, on the location and extent of the bleeding and on the patient’s clinical condition. The number of units of factor VIII administered is expressed in International Units (IU), which are related to the current WHO standard for factor VIII products. Factor VIII activity in plasma is expressed either as a percentage (relative to normal human plasma) or in international units (relative to an International Standard for factor VIII in plasma). One international unit of factor VIII activity is equivalent to that quantity of factor VIII in one ml of normal human plasma. On demand treatment The calculation of the required dosage of factor VIII is based on the empirical finding that 1 international unit factor VIII per kg body weight raises the plasma factor VIII activity by about 2 % (2 international units/dl) of normal activity. The required dosage is determined using the following formula: 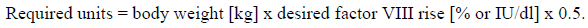 The amount to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case. In the case of the following haemorrhagic events, the factor VIII activity should not fall below the given plasma activity level (in % of normal or international units/dl) in the corresponding period. The following table can be used to guide dosing in bleeding episodes and surgery: 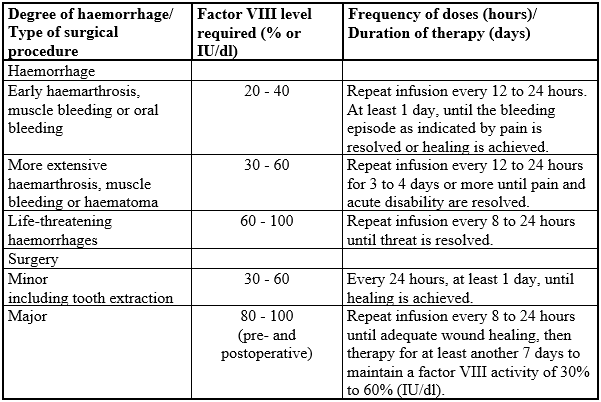 Prophylaxis For long term prophylaxis against bleeding in patients with severe haemophilia A, the usual doses are 20 to 40 international units of factor VIII per kg body weight at intervals of 2 to 3 days. In some cases, especially in younger patients, shorter dosage intervals or higher doses may be necessary. During the course of treatment, appropriate determination of factor VIII levels is advised to guide the dose to be administered and the frequency of repeated infusions. In the case of major surgical interventions in particular, a precise monitoring of the substitution therapy by means of coagulation analysis (plasma factor VIII activity) is indispensable. Individual patients may vary in their response to factor VIII, achieving different levels of in vivo recovery and demonstrating different half-lives. Patients should be monitored for the development of factor VIII inhibitors. See also section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Previously untreated patients The safety and efficacy of Beriate in previously untreated patients have not yet been established. Paediatric population There is limited data on the use of Beriate in the treatment of children less than 6 years. **Method of administration** For intravenous use. Reconstitute the product as described in section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The preparation should be warmed to room or body temperature before administration. Inject or infuse slowly intravenously at a rate which the patient finds comfortable. The injection or infusion rate should not exceed 2 ml per minute. Observe the patient for any immediate reaction. If any reaction takes place that might be related to the administration of Beriate, the rate of infusion should be decreased or the infusion stopped, as required by the clinical condition of the patient (see also section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Treatment and prophylaxis of bleeding in patients with haemophilia A (congenital factor VIII deficiency). This product may be used in the management of acquired factor VIII deficiency.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
B02BD02
coagulation factor VIII
Manufacturer Information
CSL BEHRING PTE. LTD.
CSL Behring GmbH (Manufacturer of drug product)
CSL Behring GmbH (Diluent)
Active Ingredients
Documents
Package Inserts
BERIATE POWDER AND SOLVENT FOR SOLUTION FOR INJECTION PI.pdf
Approved: October 31, 2022
