Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SUSPENSION
**4.2 Posology and method of administration** Posology FYCOMPA must be titrated, according to individual patient response, in order to optimise the balance between efficacy and tolerability. Perampanel suspension should be taken orally once daily at bedtime. It may be taken with or without food, but preferably always under the same conditions. Switching between the tablet and suspension formulation should be done with caution (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The physician should prescribe the most appropriate formulation and strength according to weight and dose. _Partial-Onset Seizures_ \[Monotherapy\] The following table summarises the recommended posology for adults, adolescents, and children from 4 years of age. More details are provided below the table. 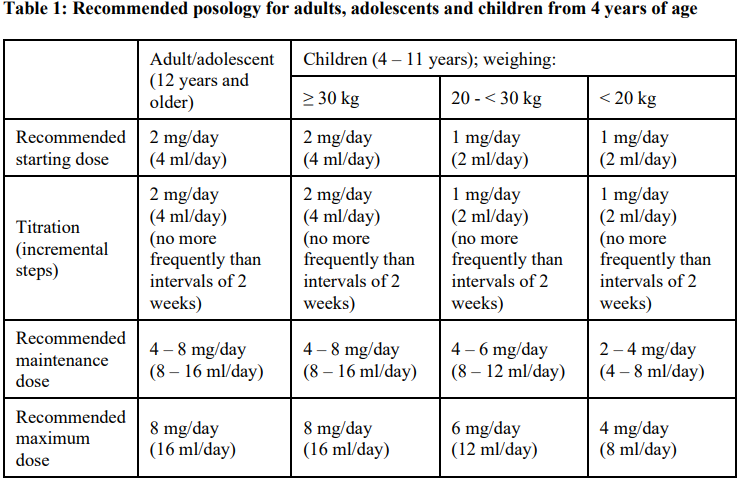 _Adults, adolescents age ≥12 years_ The starting oral dose is 2 mg once daily as perampanel at bedtime, and the daily dose may then be increased by 2 mg at intervals of 2 weeks or longer. The maintenance dose is 4–8 mg once daily. Dosage may be increased or decreased as necessary by 2 mg or less at intervals of 2 weeks or longer based on individual clinical response and tolerability, but the maximum daily dose should not be over 8 mg. _Children (from 4 to 11 years) weighing ≥30 kg_ The starting oral dose is 2 mg once daily as perampanel at bedtime, and the daily dose may then be increased by 2 mg at intervals of 2 weeks or longer. The maintenance dose is 4–8 mg once daily. Dosage may be increased or decreased as necessary by 2 mg or less at intervals of 2 weeks or longer based on individual clinical response and tolerability, but the maximum daily dose should not be over 8 mg. _Children (from 4 to 11 years of age) weighing 20 kg and <30 kg_ The starting oral dose is 1 mg once daily as perampanel at bedtime, and the daily dose may then be increased by 1 mg at intervals of 2 weeks or longer. The maintenance dose is 4–6 mg once daily. Dosage may be increased or decreased as necessary by 1 mg or less at intervals of 2 weeks or longer based on individual clinical response and tolerability, but the maximum daily dose should not be over 6 mg. _Children (from 4 to 11 years of age) weighing <20 kg_ The starting oral dose is 1 mg once daily as perampanel at bedtime, and the daily dose may then be increased by 1 mg at intervals of 2 weeks or longer. The maintenance dose is 2–4 mg once daily. Dosage may be increased or decreased as necessary by 1 mg or less at intervals of 2 weeks or longer based on individual clinical response and tolerability, but the maximum daily dose should not be over 4 mg. \[Adjunctive therapy\] Perampanel at doses of 4 mg/day to 12 mg/day has been shown to be effective therapy in partial-onset seizures. The following table summarises the recommended posology for adults, adolescents and children from 4 years of age. More details are provided below the table. 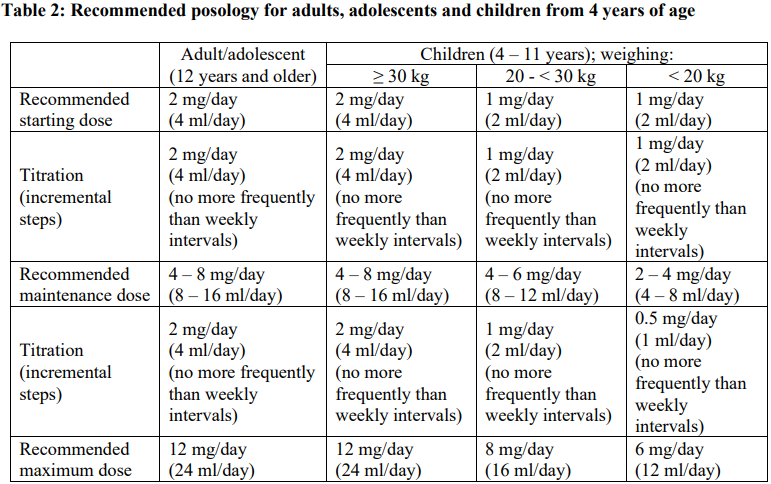 _Adults, adolescents age ≥ 12 years_ Treatment with FYCOMPA should be initiated with a dose of 2 mg/day (4 ml/day). The dose may be increased based on clinical response and tolerability by increments of 2 mg (4 ml) (either weekly or every 2 weeks as per half-life considerations described below) to a maintenance dose of 4 mg/day (8 ml/day) to 8 mg/day (16 ml/day). Depending upon individual clinical response and tolerability at a dose of 8 mg/day (16 ml/day), the dose may be increased by increments of 2 mg/day (4 ml/day) to 12 mg/day (24 ml/day). Patients who are taking concomitant medicinal products that do not shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 2-week intervals. Patients who are taking concomitant medicinal products that shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 1-week intervals. _Children (from 4 to 11 years) weighing ≥ 30 kg_ Treatment with FYCOMPA should be initiated with a dose of 2 mg/day (4 ml/day). The dose may be increased based on clinical response and tolerability by increments of 2 mg (4 ml/day) (either weekly or every 2 weeks as per half-life considerations described below) to a maintenance dose of 4 mg/day (8 ml/day) to 8 mg/day (16 ml/day). Depending upon individual clinical response and tolerability at a dose of 8 mg/day (16 ml/day), the dose may be increased by increments of 2 mg/day (4 ml/day) to 12 mg/day (24 ml/day). Patients who are taking concomitant medicinal products that do not shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 2-week intervals. Patients who are taking concomitant medicinal products that shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 1-week intervals. _Children (from 4 to 11 years of age) weighing 20 kg and < 30 kg_ Treatment with FYCOMPA should be initiated with a dose of 1 mg/day (2 ml/day). The dose may be increased based on clinical response and tolerability by increments of 1 mg (2 ml/day) (either weekly or every 2 weeks as per half-life considerations described below) to a maintenance dose of 4 mg/day (8 ml/day) to 6 mg/day (12 ml/day). Depending upon individual clinical response and tolerability at a dose of 6 mg/day (12 ml/day), the dose may be increased by increments of 1 mg/day (2 ml/day) to 8 mg/day (16 ml/day). Patients who are taking concomitant medicinal products that do not shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 2-week intervals. Patients who are taking concomitant medicinal products that shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 1-week intervals. _Children (from 4 to 11 years of age) weighing < 20 kg_ Treatment with FYCOMPA should be initiated with a dose of 1 mg/day (2 ml/day). The dose may be increased based on clinical response and tolerability by increments of 1 mg (2 ml/day) (either weekly or every 2 weeks as per half-life considerations described below) to a maintenance dose of 2 mg/day (4 ml/day) to 4 mg/day (8 ml/day). Depending upon individual clinical response and tolerability at a dose of 4 mg/day (8 ml/day), the dose may be increased by increments of 0.5 mg/day (1 ml/day) to 6 mg/day (12 ml/day). Patients who are taking concomitant medicinal products that do not shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 2-week intervals. Patients who are taking concomitant medicinal products that shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 1-week intervals. _Primary Generalised Tonic-Clonic Seizures_ Perampanel at a dose up to 8 mg/day has been shown to be effective in primary generalised tonic-clonic seizures. The following table summarises the recommended posology for adults, adolescents and children from 7 years of age. More details are provided below the table. 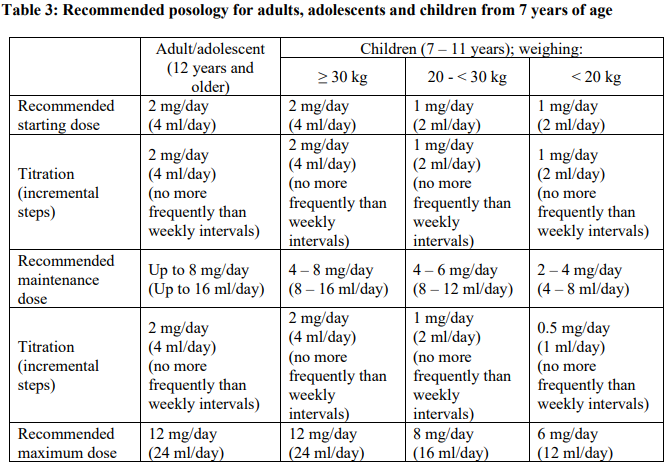 _Adults, adolescents age ≥ 12 years_ Treatment with FYCOMPA should be initiated at a dose of 2 mg/day (4 ml/day). The dose may be increased based on clinical response and tolerability by increments of 2 mg (4 ml/day) (either weekly or every 2 weeks, as per half-life considerations described below) to a maintenance dose of up to 8 mg/day (16 ml/day). Depending upon individual clinical response and tolerability at a dose of 8 mg/day (16 ml/day), the dose may be increased up to 12 mg/day (24 ml/day), which may be effective in some patients (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients who are taking concomitant medicinal products that do not shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 2-week intervals. Patients who are taking concomitant medicinal products that shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 1-week intervals. _Children (from 7 to 11 years) weighing ≥ 30 kg_ Treatment with FYCOMPA should be initiated with a dose of 2 mg/day (4 ml/day). The dose may be increased based on clinical response and tolerability by increments of 2 mg (4 ml) (either weekly or every 2 weeks as per half-life considerations described below) to a maintenance dose of 4 mg/day (8 ml/day) to 8 mg/day (16 ml/day). Depending upon individual clinical response and tolerability at a dose of 8 mg/day (16 ml/day), the dose may be increased by increments of 2 mg/day (4 ml/day) to 12 mg/day (24 ml/day). Patients who are taking concomitant medicinal products that do not shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 2-week intervals. Patients who are taking concomitant medicinal products that shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 1-week intervals. _Children (from 7 to 11 years of age) weighing 20 kg and < 30 kg_ Treatment with FYCOMPA should be initiated with a dose of 1 mg/day (2 ml/day). The dose may be increased based on clinical response and tolerability by increments of 1 mg (2 ml) (either weekly or every 2 weeks as per half-life considerations described below) to a maintenance dose of 4 mg/day (8 ml/day) to 6 mg/day (12 ml/day). Depending upon individual clinical response and tolerability at a dose of 6 mg/day, the dose may be increased by increments of 1 mg/day (2 ml/day) to 8 mg/day (16 ml/day). Patients who are taking concomitant medicinal products that do not shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 2-week intervals. Patients who are taking concomitant medicinal products that shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 1-week intervals. _Children (from 7 to 11 years of age) weighing < 20 kg_ Treatment with FYCOMPA should be initiated with a dose of 1 mg/day (2 ml/day). The dose may be increased based on clinical response and tolerability by increments of 1 mg (2 ml) (either weekly or every 2 weeks as per half-life considerations described below) to a maintenance dose of 2 mg/day (4 ml/day) to 4 mg/day (8 ml/day). Depending upon individual clinical response and tolerability at a dose of 4 mg/day (8 ml/day), the dose may be increased by increments of 0.5 mg/day (1 ml/day) to 6 mg/day (12 ml/day). Patients who are taking concomitant medicinal products that do not shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 2-week intervals. Patients who are taking concomitant medicinal products that shorten the half-life of perampanel (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be titrated no more frequently than at 1-week intervals. _Paediatric population_ The safety and efficacy of perampanel have not yet been established in children below 4 years of age in the POS indication or in children below 7 years of age in the PGTCS indication. _Elderly (65 years of age and above)_ Clinical studies of FYCOMPA in epilepsy did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Analysis of safety information in 905 perampanel-treated elderly patients (in double-blind studies conducted in non-epilepsy indications) revealed no age-related differences in the safety profile. In combination with the lack of age-related difference in perampanel exposure, the results indicate that dose-adjustment in the elderly is not required. Perampanel should be used with caution in elderly taking into account the drug interaction potential in polymedicated patients (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ Dose adjustment is not required in patients with mild renal impairment. Use in patients with moderate or severe renal impairment or patients undergoing haemodialysis is not recommended. _Hepatic impairment_ Dose increases in patients with mild and moderate hepatic impairment should be based on clinical response and tolerability. For patients with mild or moderate hepatic impairment, dosing can be initiated at 2 mg (4 ml). Patients should be up-titrated using 2 mg (4 ml) doses no faster than every 2 weeks based on tolerability and effectiveness. Perampanel dosing for patients with mild and moderate impairment should not exceed 8 mg. Use in patients with severe hepatic impairment is not recommended. _Missed Dose_ Single missed dose: As FYCOMPA has a long half-life, the patient should wait and take their next dose as scheduled. If more than 1 dose has been missed, for a continuous period of less than 5 half-lives (3 weeks for patients not taking FYCOMPA metabolism-inducing anti-epileptic drugs (AED), 1 week for patients taking FYCOMPA metabolism-inducing AEDs consideration should be given to restart treatment from the last dose level. If a patient has discontinued FYCOMPA for a continuous period of more than 5 half-lives, it is recommended that initial dosing recommendations given above should be followed. _Withdrawal_ It is recommended that discontinuation be undertaken gradually to minimise the potential for rebound seizures. However, due to its long half-life and subsequent slow decline in plasma concentrations, perampanel can be discontinued abruptly if absolutely needed. Method of administration FYCOMPA is for oral use. Before use, shake the bottle for at least 5 seconds. Preparation: The press-in-bottle adapter (PIBA) which is supplied in the product carton should be inserted firmly into the neck of the bottle before use and remain in place for the duration of the usage of the bottle. The oral syringe should be inserted into the PIBA and the dose withdrawn from the inverted bottle. The cap should be replaced after each use. The cap fits properly when the PIBA is in place.
ORAL
Medical Information
**4.1 Therapeutic indications** FYCOMPA is indicated for: - treatment of partial-onset seizures (POS) with or without secondarily generalised seizures in patients from 4 years of age and older. - adjunctive treatment of primary generalised tonic-clonic (PGTC) seizures in patients from 7 years of age and older with idiopathic generalised epilepsy (IGE).
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
N03AX22
perampanel
Manufacturer Information
EISAI (SINGAPORE) PTE. LTD.
Delpharm Huningue S.A.S.
Active Ingredients
Documents
Package Inserts
Fycompa OS_PI.pdf
Approved: July 4, 2023
