Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SUSPENSION
**4.2 Posology and method of administration** Posology Adverse drug reactions may be reduced by using the lowest effective dose for the shortest duration possible to manage the symptoms (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Adults_ Usually, 250–500 mg (10–20 ml) twice a day based on the individual need. If the predominant symptom in rheumatoid arthritis is morning stiffness, a single dose of 500–750 mg (20–30 ml) in the evenings may be adequate. In the treatment of acute gout, the recommended dose is 750 mg at once then 250 mg every 8 hours until the attack has passed. In the treatment of acute musculoskeletal disorders and dysmenorrhoea the recommended dose is 500 mg initially followed by 250 mg at 6–8 hour intervals as needed, with a maximum daily dose after the first day of 1 250 mg. _Paediatric population (over 5 years):_ For juvenile rheumatoid arthritis: in children aged over 5 years, the recommended daily dose is 10 mg/kg divided into two doses. Dosing as per the table below. Patients weighing over 50 kg may be administered the adult dosage. 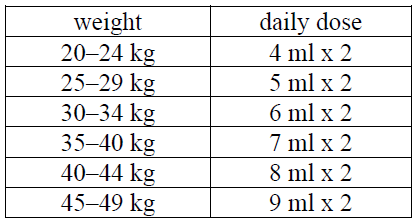 _Elderly patients_ In those over 70, the concentration of free naproxen in plasma is higher than in younger persons, and the elimination of naproxen is slower. Elderly patients may be more susceptible than others to the adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs). For this reason, the lower single doses described above, i.e. 250 mg (10 ml) twice daily, are recommended for elderly patients. _Renal impairment_ The lowest effective dose should be used in patients with mild renal insufficiency, and renal function should be monitored. If possible, the use of Pronaxen oral suspension should be avoided in patients with moderate (creatinine clearance 50 to 10 ml/min or serum creatinine 160 to 565 micromol/l) or severe renal insufficiency (creatinine clearance <10 ml/min or serum creatinine >565 micromol/l) (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment_ Pronaxen oral suspension should be used with caution in patients with hepatic insufficiency (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If possible, the use of Pronaxen oral suspension should be avoided in patients with severe hepatic insufficiency or a cirrhotic hepatic condition. Method of administration For instructions on reconstitution of the medicinal product before administration of the medicine, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
ORAL
Medical Information
**4.1 Therapeutic indications** Adults - Rheumatoid arthritis, spondyloarthropathies (including ankylosing spondylitis) - Osteoarthrosis - Acute gout - Acute musculoskeletal disorders with pain - Dysmenorrhoea Children - Juvenile rheumatoid arthritis
**4.3 Contraindications** - Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ - Asthma and allergy if the patient develops hypersensitivity symptoms in connection with the use of acetylsalicylic acid (ASA) or other NSAIDs - Third trimester of pregnancy - Severe cardiac insufficiency - A history of gastrointestinal bleeding or perforation, related to the use of NSAIDs - Acute gastric/duodenal ulcer or associated bleeding or history of recurrent episodes (at least two separate, confirmed episodes) - Other conditions predisposing to gastrointestinal bleeding.
M01AE02
naproxen
Manufacturer Information
ORION PHARMA (SG) PTE. LTD.
Orion Corporation, Orion Pharma
Active Ingredients
Documents
Package Inserts
Pronaxen Oral Suspension PI.pdf
Approved: March 21, 2023
