Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION, CONCENTRATE
**POSOLOGY AND METHOD OF ADMINISTRATION** _**Recommended dosage**_ For breast, non-small cell lung, gastric, and head and neck cancers, premedication consisting of an oral corticosteroid, such as dexamethasone 16 mg per day (e.g. 8 mg BID) for 3 days starting 1 day prior to docetaxel administration, unless contraindicated, can be used (see section on Special warnings and Precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Prophylactic G-CSF may be used to mitigate the risk of hematological toxicities. For prostate cancer, given the concurrent use of prednisone or prednisolone the recommended premedication regimen is oral dexamethasone 8 mg, 12 hours, 3 hours and 1 hour before the docetaxel infusion (see section Special warnings and Precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Docetaxel is administered as a one-hour infusion every three weeks. _Breast cancer_ For the treatment of patients with locally advanced or metastatic breast cancer, the recommended dosage of docetaxel is 100 mg/m2 in monotherapy. In first-line treatment, docetaxel 60 mg/m2 is given in combination therapy with doxorubicin (50 mg/m2). In combination with trastuzumab the recommended dose of docetaxel is 100 mg/m2 every three weeks, with trastuzumab administered weekly. In the pivotal trial the initial docetaxel infusion was started the day following the first dose of trastuzumab. The subsequent docetaxel doses were administered immediately after completion of the trastuzumab infusion, if the preceding dose of trastuzumab was well tolerated. For trastuzumab dosage and administration, see trastuzumab package insert – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. In combination with capecitabine, the recommended dose of docetaxel is 75 mg/m2 every three weeks, combined with capecitabine at 1250 mg/m2 twice daily (within 30 minutes after a meal) for 2 weeks followed by 1-week rest period. For capecitabine dose calculation according to body surface area, see capecitabine package insert. _Non-small cell lung cancer_ In chemotherapy naïve patients treated for non-small cell lung cancer, the recommended dose regimen is docetaxel 75 mg/m2 immediately followed by cisplatin 75 mg/m2 over 30–60 minutes. For treatment after failure of prior platinum-based chemotherapy, the recommended dosage is 75 mg/m2 as a single agent. _Prostate cancer_ The recommended dose of docetaxel is 75 mg/m2. Prednisone or prednisolone 5 mg orally twice daily is administered continuously (see section on Pharmacodynamic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Gastric adenocarcinoma_ The recommended dose of docetaxel is 75 mg/m2 as a 1 hour infusion, followed by cisplatin 75 mg/m2, as a 1 to 3 hour infusion (both on day 1 only), followed by 5-fluorouracil 750 mg/m2 per day given as a 24-hour continuous infusion for 5 days, starting at the end of the cisplatin infusion. Treatment is repeated every three weeks. Patients must receive premedication with antiemetics and appropriate hydration for cisplatin administration. Prophylactic G-CSF should be used to mitigate the risk of hematological toxicities (See also Dosage adjustments during treatment). _Head and neck cancer_ Patients must receive premedication with antiemetics and appropriate hydration (prior to and after cisplatin administration). Prophylactic G-CSF may be used to mitigate the risk of hematological toxicities. All patients on the docetaxel-containing arm of the TAX 323 and TAX 324 studies, received prophylactic antibiotics. - Induction chemotherapy followed by radiotherapy (TAX 323) For the induction treatment of inoperable locally advanced squamous cell carcinoma of the head and neck (SCCHN), the recommended dose of docetaxel is 75 mg/m2 as a 1 hour infusion followed by cisplatin 75 mg/m2 over 1 hour, on day one, followed by 5-fluorouracil as a continuous infusion at 750 mg/m2 per day for five days. This regimen is administered every 3 weeks for 4 cycles. Following chemotherapy, patients should receive radiotherapy. - Induction chemotherapy followed by chemoradiotherapy (TAX 324) For the induction treatment of patients with locally advanced (technically unresectable, low probability of surgical cure, and aiming at organ preservation) squamous cell carcinoma of the head and neck (SCCHN), the recommended dose of docetaxel is 75 mg/m2 as a 1 hour intravenous infusion on day 1, followed by cisplatin 100 mg/m2 administered as a 30-minute to 3-hour infusion, followed by 5-fluorouracil 1000 mg/m2 /day as a continuous infusion from day 1 to day 4. This regimen is administered every 3 weeks for 3 cycles. Following chemotherapy, patients should receive chemoradiotherapy. For cisplatin and 5-fluorouracil dose modifications, see the corresponding package insert. **_Dosage adjustments during treatment_** _General_ Docetaxel should be administered when the neutrophil count is ≥ 1,500 cells/mm3. In patients who experienced either febrile neutropenia, neutrophil count < 500 cells/mm3 for more than one week, severe or cumulative cutaneous reactions or severe peripheral neuropathy during docetaxel therapy, the dose of docetaxel should be reduced from 100 mg/m2 to 75 mg/m2 and/or from 75 to 60 mg/m2. If the patient continues to experience these reactions at 60 mg/m2, the treatment should be discontinued. _In combination with cisplatin_ For patients who are dosed initially at docetaxel 75 mg/m2 in combination with cisplatin and whose nadir of platelet count during the previous course of therapy is < 25000 cells/mm3, or in patients who experience febrile neutropenia, or in patients with serious non-hematologic toxicities, the docetaxel dosage in subsequent cycles should be reduced to 65 mg/m2. For cisplatin dosage adjustments, see manufacturer’s package insert. _In combination with capecitabine_ - For capecitabine dose modifications, see capecitabine package insert. - For patients developing the first appearance of a Grade 2 toxicity, which persists at the time of the next docetaxel/capecitabine treatment, delay treatment until resolved to Grade 0–1, and resume at 100% of the original dose. - For patients developing the second appearance of a Grade 2 toxicity, or the first appearance of a Grade 3 toxicity, at any time during the treatment cycle, delay treatment until resolved to Grade 0–1, then resume treatment with docetaxel 55 mg/m2. - For any subsequent appearances of toxicities, or any Grade 4 toxicities, discontinue the docetaxel dose. For trastuzumab dose modifications, see trastuzumab package insert. _In combination with cisplatin and 5-fluorouracil:_ If an episode of febrile neutropenia, prolonged neutropenia or neutropenic infection occurs despite G-CSF use, the docetaxel dose should be reduced from 75 to 60 mg/m2. If subsequent episodes of complicated neutropenia occur the docetaxel dose should be reduced from 60 to 45 mg/m2. In case of Grade 4 thrombocytopenia the docetaxel dose should be reduced from 75 to 60 mg/m2. Patients should not be retreated with subsequent cycles of docetaxel until neutrophils recover to a level > 1,500 cells/mm3 and platelets recover to a level > 100,000 cells/mm3. Discontinue treatment if these toxicities persist. (See section on special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Recommended dose modifications for gastrointestinal toxicities in patients treated with docetaxel in combination with cisplatin and 5-fluorouracil (5-FU): 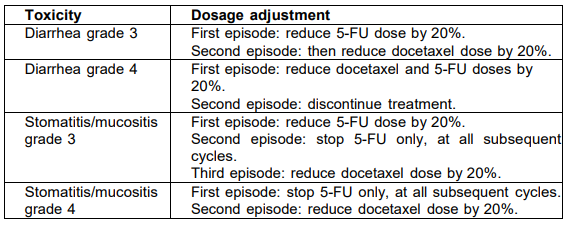 For cisplatin and 5-fluorouracil dose adjustments, see the corresponding package insert. In the pivotal SCCHN trials patients who experienced complicated neutropenia (including prolonged neutropenia, febrile neutropenia, or infection), it was recommended to use G-CSF to provide prophylactic coverage (eg, day 6–15) in all subsequent cycles. _**Special populations**_ _Patients with hepatic impairment_ Based on pharmacokinetic data with docetaxel at 100 mg/m2 as single agent, patients who have both elevations of transaminase (ALT and/or AST) greater than 1.5 times the upper limit of the normal range (ULN) and alkaline phosphatase greater than 2.5 times the ULN, the recommended dose of docetaxel is 75 mg/m2 (see sections Special warnings and precautions for use and Pharmacokinetic properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For those patients with serum bilirubin > ULN and/or ALT and AST > 3.5 times the ULN associated with alkaline phosphatase > 6 times the ULN, no dose-reduction can be recommended and docetaxel should not be used unless strictly indicated. In combination with cisplatin and 5-fluorouracil for the treatment of patients with gastric adenocarcinoma, the pivotal clinical trial excluded patients with ALT and/or AST > 1.5 × ULN associated with alkaline phosphatase > 2.5 × ULN, and bilirubin > 1 x ULN; for these patients, no dose-reductions can be recommended and docetaxel should not be used unless strictly indicated. No data are available in patients with hepatic impairment treated by docetaxel in combination in the other indications. This medicinal product contains 400 mg ethanol per ml concentrate. This has to be taken into account in high-risk groups such as patients with liver disease. _Children and adolescents_ Docetaxel is not recommended for use in children due to insufficient data on safety and/or efficacy. _Elderly_ Based on a population pharmacokinetic analysis, there are no special instructions for use in the elderly. In combination with capecitabine, for patients 60 years of age or more, a starting dose reduction of capecitabine to 75% is recommended (see capecitabine package insert).
INTRAVENOUS
Medical Information
**INDICATIONS** _**Breast cancer**_ Docetaxel STADA® is indicated for the treatment of patients with locally advanced or metastatic breast carcinoma. **_Non-small cell lung cancer_** Docetaxel STADA® is indicated for the treatment of patients with locally advanced or metastatic non-small cell lung cancer. _**Prostate cancer**_ Docetaxel STADA® in combination with prednisone or prednisolone is indicated for the treatment of patients with hormone refractory metastatic prostate cancer. _**Gastric Adenocarcinoma**_ Docetaxel STADA® in combination with cisplatin and 5-fluorouracil is indicated for the treatment of patients with metastatic gastric adenocarcinoma, including adenocarcinoma of the gastroesophageal junction, who have not received prior chemotherapy for metastatic disease. _**Head and neck cancer**_ Docetaxel STADA® in combination with cisplatin and 5-fluorouracil is indicated for the induction treatment of patients with locally advanced squamous cell carcinoma of the head and neck.
**CONTRAINDICATIONS** Hypersensitivity to the active substance or to any of the excipients. Patients with baseline neutrophil count of < 1,500 cells/mm3. Patients with severe liver impairment (see sections Method of administration and Special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Contraindications for other medicinal products also apply, when combined with docetaxel.
L01CD02
docetaxel
Manufacturer Information
DCH AURIGA SINGAPORE
Actavis Italy S.p.A. (Nerviano Plant)
Active Ingredients
Documents
Package Inserts
Docetaxel Stada Injection PI.pdf
Approved: October 12, 2022
