Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Dosage and Administration** CAELYX exhibits unique pharmacokinetic properties and must not be used interchangeably with other formulations of doxorubicin hydrochloride. CAELYX should only be administered under the supervision of a qualified oncologist specialized in the administration of cytotoxic agents. **Breast/Ovarian Cancer** CAELYX is administered intravenously at a dose of 50 mg/m2 once every four weeks for as long as the disease does not progress and the patient continues to tolerate treatment. **Multiple myeloma** CAELYX is administered at 30 mg/m2 on day 4 of the bortezomib 3 week regimen as a 1 hour infusion administered immediately after the bortezomib infusion. The bortezomib regimen consists of 1.3 mg/m2 on days 1, 4, 8, and 11 every 3 weeks. The dose should be repeated as long as patients respond satisfactorily and tolerate treatment. **AIDS-KS patients** CAELYX should be administered intravenously at 20mg/m2 every two-to-three weeks. Intervals shorter than 10 days should be avoided as drug accumulation and increased toxicity cannot be ruled out. Patients should be treated for two-to-three months to achieve a therapeutic response. Treatment should be continued as needed to maintain a therapeutic response. **Guidelines for CAELYX Dose Modification** To manage adverse events such as palmar-plantar erythrodysesthesia (PPE), stomatitis or hematologic toxicity, the dose may be reduced or delayed. Guidelines for CAELYX dose modification secondary to these adverse effects are provided in the tables below. The toxicity grading in these tables is based on the National Cancer Institute Common Toxicity Criteria (NCI-CTC). The tables for PPE (Table 1) and stomatitis (Table 2) provide the schedule followed for dose modification in clinical trials in the treatment of breast or ovarian cancer (modification of the recommended 4 week treatment cycle): If these toxicities occur in patients with AIDS-related KS, the recommended 2 to 3 week treatment cycle can be modified in a similar manner. The table for hematologic toxicity (Table 3) provides the schedule followed for dose modification in clinical trials in the treatment of patients with breast or ovarian cancer only. Dose modification in patients with AIDS-KS is addressed in _Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.  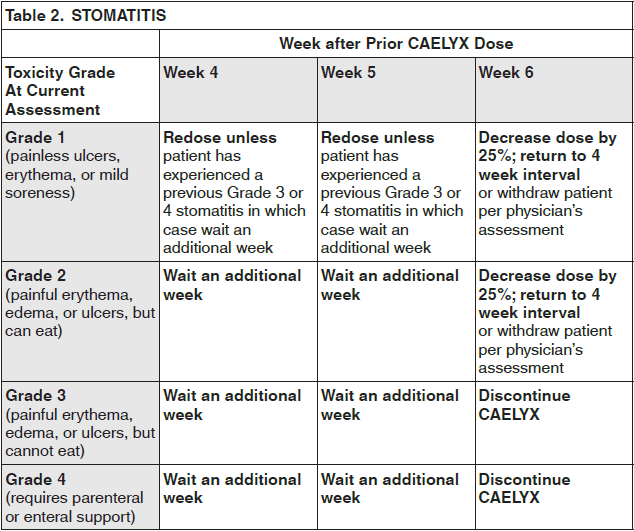 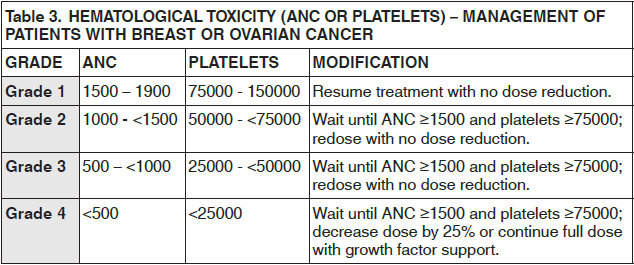 For multiple myeloma patients treated with CAELYX in combination with bortezomib who experience PPE or stomatitis, the CAELYX dose should be modified as described in Table 1 and 2 above respectively. For more detailed information on bortezomib dosing and dosage adjustments, see the prescribing information for bortezomib. 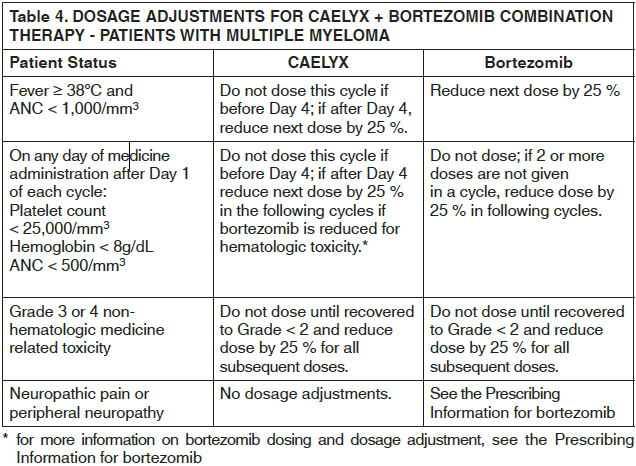 _**Pediatric patients**_ Limited Phase I safety data indicate that doses up to 60 mg/m2 every 4 weeks are well tolerated in pediatric patients; however, effectiveness in patients less than 18 years of age has not been established. _**Elderly patients**_ Population-based analysis demonstrates that age across the range tested (21–75 years) does not significantly alter the pharmacokinetics of CAELYX. _**Patients with impaired renal function**_ As doxorubicin is metabolised by the liver and excreted in the bile, dose modification should not be required with CAELYX. Population-based analysis confirms that changes in renal function over the range tested (estimated creatinine clearance 30–156 mL/min) do not alter the pharmacokinetics of CAELYX. No pharmacokinetic data are available in patients with creatinine clearance of less than 30 mL/min. _**Patients with impaired hepatic function**_ CAELYX pharmacokinetics determined in a small number of patients with elevated total bilirubin levels do not differ from patients with normal total bilirubin; however, until further experience is gained, the CAELYX dosage in patients with impaired hepatic function should be reduced based on the experience from the breast and ovarian clinical trial programs as follows: at initiation of therapy, if the bilirubin is between 1.2 – 3.0 mg/dL, the first dose is reduced by 25%. If the bilirubin is > 3.0 mg/dL, the first dose is reduced by 50%. If the patient tolerates the first dose without an increase in serum bilirubin or liver enzymes, the dose for cycle 2 can be increased to the next dose level, i.e., if reduced by 25% for the first dose, increase to full dose for cycle 2; if reduced by 50% for the first dose, increase to 75% of full dose for cycle 2. The dosage can be increased to full dose for subsequent cycles if tolerated. CAELYX can be administered to patients with liver metastases with concurrent elevation of bilirubin and liver enzymes up to 4 × the upper limit of the normal range. Prior to CAELYX administration, evaluate hepatic function using conventional clinical laboratory tests such as ALT/AST, alkaline phosphatase, and bilirubin. _**AIDS-KS patients with splenectomy**_ As there is no experience with CAELYX in patients with splenectomy, treatment with CAELYX is not recommended. **Administration** For doses <90 mg: dilute CAELYX in 250 mL Dextrose 5 % in Water. For doses ≥90 mg: dilute CAELYX in 500 mL Dextrose 5 % in Water. If the patient experiences early symptoms or signs of infusion reaction, immediately discontinue the infusion, give appropriate premedications (antihistamine and/or short acting corticosteroid) and restart at a slower rate. DO NOT administer as a bolus injection or undiluted dispersion. It is recommended that the CAELYX infusion line be connected through the side port of an intravenous infusion of Dextrose 5% in Water to achieve further dilution and minimize the risk of thrombosis and extravasation. The infusion may be given through a peripheral vein. CAELYX must not be given by the intramuscular or subcutaneous route. Do not use with in-line filters. **Breast cancer/Ovarian cancer** To minimize the risk of infusion reactions, the initial dose is administered at a rate no greater than 1 mg/minute. If no infusion reaction is observed, subsequent CAELYX infusions may be administered over a 60-minute period. In the breast cancer trial program, modification of the infusion was permitted for those patients experiencing an infusion reaction as follows: 5 % of the total dose was infused slowly over the first 15 minutes. If tolerated without reaction, the infusion rate was doubled for the next 15 minutes. If tolerated, the infusion was completed over the next hour for a total infusion time of 90 minutes. Subsequent CAELYX infusions may be administered over a 60 minute period. **Multiple myeloma** The intravenous catheter and tubing should be flushed with 5% glucose solution for infusion between administration of CAELYX and bortezomib. Day 4 dosing of both medicinal products may be delayed up to 48 hours as medically necessary. Doses of bortezomib should be at least 72 hours apart. The first infusion of CAELYX should be administered over 90 minutes, as follows: 1. 10 mL over first 10 minutes 2. 20 mL over next 10 minutes 3. 40 mL over next 10 minutes 4. then, complete the infusion over a total of 90 minutes. Subsequent doses of CAELYX will be administered over 1 hour, as tolerated. If an infusion reaction to CAELYX occurs, stop the infusion and after the symptoms resolve, attempt to administer the remaining CAELYX over 90 minutes, as follows: 1. 10 mL over first 10 minutes 2. 20 mL over next 10 minutes 3. 40 mL over next 10 minutes 4. then, complete the remaining infusion over a total of 90 minutes. Infusion may be given through a peripheral vein or a central line. **AIDS-KS patients** CAELYX, diluted in 250 mL Dextrose 5% in Water, is administered by intravenous infusion over 30 minutes.
INTRAVENOUS
Medical Information
**Indications** **Breast Cancer** CAELYX, as monotherapy, is indicated for the treatment of metastatic breast cancer. **Ovarian Cancer** CAELYX is indicated for the treatment of advanced ovarian cancer in women who have failed a first-line platinum based chemotherapy regimen. **Multiple Myeloma** CAELYX is indicated in combination with bortezomib for the treatment of progressive multiple myeloma in patients who have received at least one prior therapy and have not previously received bortezomib. Patients should have already undergone or are unsuitable for bone marrow transplant. **AIDS-related Kaposi’s Sarcoma** CAELYX is also indicated for AIDS-related Kaposi’s sarcoma (KS) in patients with low CD4 counts (<200 CD4 lymphocytes/mm3) and extensive mucocutaneous or visceral disease. CAELYX may be used as first-line systemic chemotherapy, or as second line chemotherapy in AIDS-KS patients with disease that has progressed with, or in patients intolerant to, prior combination systemic chemotherapy comprising at least two of the following agents: a vinca alkaloid, bleomycin and standard doxorubicin (or other anthracyclines).
**Contraindications** CAELYX is contraindicated in patients who have hypersensitivity reactions to its components or to doxorubicin HCl. CAELYX should not be administered during pregnancy or while breast-feeding. CAELYX should not be used to treat AIDS-KS that may be effectively treated with local therapy or systemic alfa-interferon.
L01DB01
doxorubicin
Manufacturer Information
BAXTER HEALTHCARE (ASIA) PTE LTD
Glaxosmithkline Manufacturing S.p.A. (Parma, Italy)
Active Ingredients
Documents
Package Inserts
Caelyx_PI_Approved.pdf
Approved: June 24, 2022
