Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED, EXTENDED RELEASE
**6\. Dosage and Administration** CONTRAVE dosing should be escalated according to the following schedule: 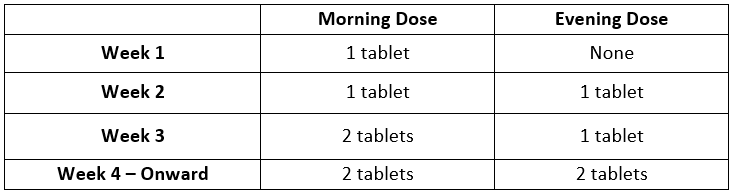 A total daily dosage of two CONTRAVE 8 mg/90 mg tablets twice daily (32 mg/360 mg) is reached at the start of Week 4. CONTRAVE should be taken by mouth in the morning and in the evening. The tablets should not be cut, chewed, or crushed. Total daily doses greater than 32 mg/360 mg per day (two tablets twice daily) are not recommended. In clinical trials, CONTRAVE was administered with meals. However, CONTRAVE should not be taken with a high-fat meal because of a resulting significant increase in bupropion and naltrexone systemic exposure _\[see Warnings and Precautions and Clinical Pharmacology_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Patients may develop elevated blood pressure or heart rate during CONTRAVE treatment; the risk may be greater during the initial three months of therapy _\[see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Because patients with hypertension may be at increased risk for developing blood pressure elevations, such patients should be monitored for this potential effect when initiating treatment with CONTRAVE. Response to therapy should be evaluated after 12 weeks at the maintenance dosage. If a patient has not lost at least 5% of baseline body weight, discontinue CONTRAVE, as it is unlikely that the patient will achieve and sustain clinically meaningful weight loss with continued treatment. The need for continued treatment should be re-evaluated annually. The product is only for the intended population. Refer to the Contraindications and Warnings and Precautions to note the patient characteristics that place patients at higher risk of adverse reactions to CONTRAVE, to help ensure appropriate patient selection – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. BMI is calculated by dividing weight (in kg) by height (in meters) squared. A BMI chart for determining BMI based on height and weight is provided in _Table 6_. 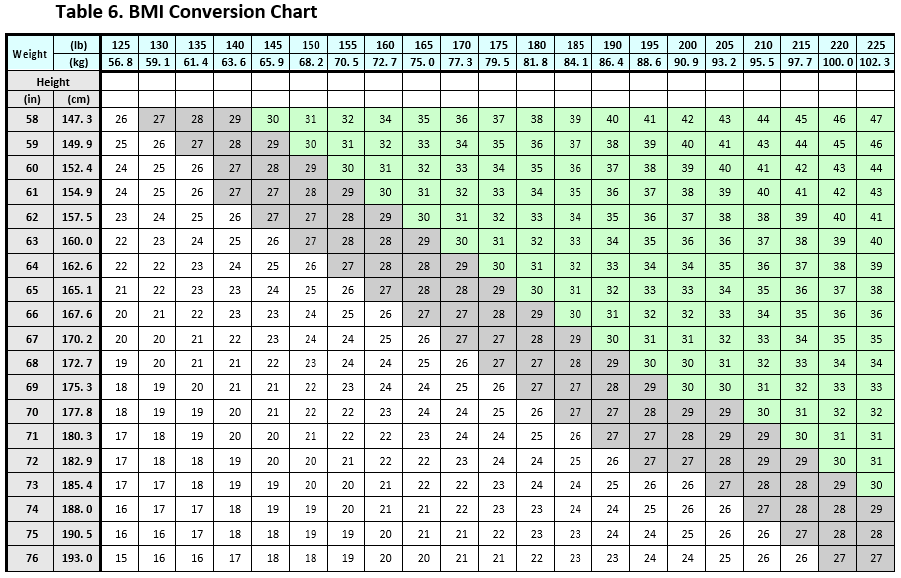 **Dose Adjustment in Patients with Renal Impairment** In patients with moderate or severe renal impairment, the maximum recommended daily dose for CONTRAVE is two tablets (one tablet each morning and evening). No dose adjustment is required in patients with mild renal impairment. CONTRAVE is not recommended for use in patients with end-stage renal disease _\[see Use in Specific Population and Clinical Pharmacology_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **Dose Adjustment in Patients with Hepatic Impairment** CONTRAVE is contraindicated in patients with severe hepatic impairment. It is not recommended in patients with moderate hepatic impairment. In patients with mild hepatic impairment, the maximum recommended daily dose for CONTRAVE is two tablets (one tablet in the morning and one tablet in the evening). It is recommended that patients with mild hepatic impairment initiate treatment with one tablet in the morning for the first week of treatment, and escalate to one tablet in the morning and one tablet in the evening from week 2 onwards. Degree of hepatic impairment should be assessed using the Child-Pugh score _\[see Use in Specific Population and Clinical Pharmacology_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Antidepressant** At least 14 days should elapse between discontinuation of an MAOI intended to treat depression and initiation of therapy with CONTRAVE. Conversely, at least 14 days should be allowed after stopping CONTRAVE before starting an MAOI antidepressant _\[see Contraindications and Drug Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **Concomitant Use with CYP2B6 Inhibitors** During concomitant use with CYP2B6 inhibitors (e.g., ticlopidine or clopidogrel), the maximum recommended daily dose of CONTRAVE is two tablets (one tablet each morning and evening) _\[see Drug Interactions and Clinical Pharmacology_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
ORAL
Medical Information
**5\. Indication** CONTRAVE is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index (BMI) of: - 30 kg/m2 or greater (obese) or - 27 kg/m2 or greater (overweight) in the presence of at least one weight-related comorbid condition (e.g., hypertension, type 2 diabetes mellitus, or dyslipidemia). Limitations of Use: - The effect of CONTRAVE on cardiovascular morbidity and mortality has not been established (see Warnings and Precautions, Increase in Blood Pressure and Heart Rate – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - The safety and effectiveness of CONTRAVE in combination with other products intended for weight loss, including prescription drugs, over-the-counter drugs, and herbal preparations, have not been established.
**7\. Contraindications** CONTRAVE is contraindicated in - Uncontrolled hypertension _\[see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Seizure disorder or a history of seizures _\[see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Use of other bupropion-containing products - Bulimia or anorexia nervosa, which increase the risk for seizure _\[see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Chronic opioid or opiate agonist (e.g., methadone) or partial agonists (e.g., buprenorphine) use, or acute opiate withdrawal _\[see Warnings and Precautions and Drug Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Patients undergoing an abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs _\[see Warnings and Precautions and Drug Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Concomitant administration of monoamine oxidase inhibitors (MAOI). At least 14 days should elapse between discontinuation of MAOI and initiation of treatment with CONTRAVE. There is an increased risk of hypertensive reactions when CONTRAVE is used concomitantly with MAOIs. Starting CONTRAVE in a patient treated with reversible MAOIs such as linezolid or intravenous methylene blue is also contraindicated _\[see Dosage and Administration, Drug Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Known allergy to bupropion, naltrexone or any other component of CONTRAVE. Anaphylactoid/anaphylactic reactions and Stevens-Johnson syndrome have been reported with bupropion _\[see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Patients with severe hepatic impairment _\[see Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_
A08AA62
bupropion and naltrexone
Manufacturer Information
INOVA PHARMACEUTICALS (SINGAPORE) PTE. LIMITED
Patheon Inc.
Active Ingredients
Documents
Package Inserts
Contrave PI.docx
Approved: January 3, 2022
