Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**DOSAGE AND ADMINISTRATION** **Acute Exposure to Blood Containing HBsAg(16)** Table 1 summarizes prophylaxis for percutaneous (needlestick or bite), ocular, or mucous-membrane exposure to blood according to the source of exposure and vaccination status of the exposed person. For greatest effectiveness, passive prophylaxis with Hepatitis B Immune Globulin (Human) should be given as soon as possible after exposure (its value beyond 7 days of exposure is unclear). If Hepatitis B Immune Globulin (Human) is indicated (see Table 1), an injection of 0.06 mL/kg of body weight should be administered intramuscularly (see PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) as soon as possible after exposure and within 24 hours, if possible. Consult Hepatitis B Vaccine package insert for dosage information regarding that product. 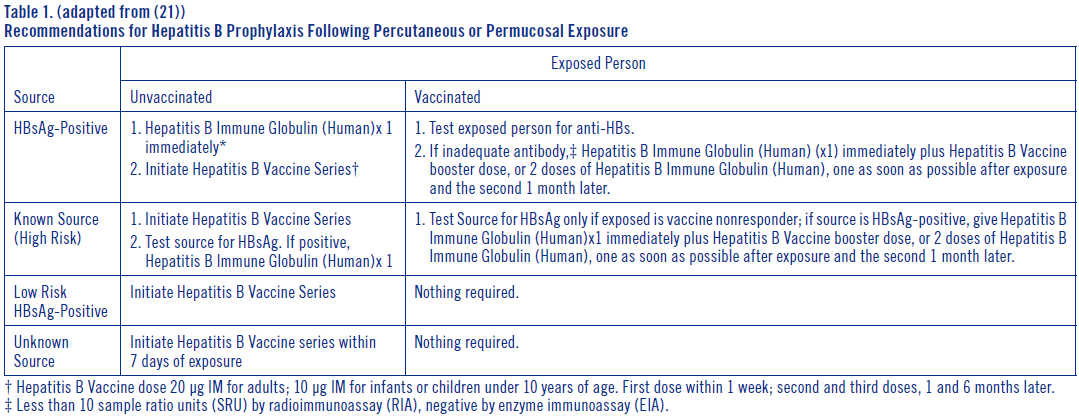 For persons who refuse Hepatitis B Vaccine, a second dose of Hepatitis B Immune Globulin (Human) should be given 1 month after the first dose. **Prophylaxis of Infants Born to HBsAg and HBeAg Positive Mothers** Efficacy of prophylactic Hepatitis B Immune Globulin (Human) in infants at risk depends on administering Hepatitis B Immune Globulin (Human) on the day of birth. It is therefore vital that HBsAg-positive mothers be identified before delivery. Hepatitis B Immune Globulin (Human) (0.5 mL) should be administered intramuscularly (IM) to the newborn infant after physiologic stabilization of the infant and preferably within 12 hours of birth. Hepatitis B Immune Globulin (Human) efficacy decreases markedly if treatment is delayed beyond 48 hours. Hepatitis B Vaccine should be administered IM in three doses of 0.5 mL of vaccine (10 mcg) each. The first dose should be given within 7 days of birth and may be given concurrently with Hepatitis B Immune Globulin (Human) but at a separate site. The second and third doses of vaccine should be given 1 month and 6 months, respectively, after the first. If administration of the first dose of Hepatitis B Vaccine is delayed for as long as 3 months, then a 0.5 mL dose of Hepatitis B Immune Globulin (Human) should be repeated at 3 months. If Hepatitis B Vaccine is refused, the 0.5 mL dose of Hepatitis B Immune Globulin (Human) should be repeated at 3 and 6 months. Hepatitis B Immune Globulin (Human) administered at birth should not interfere with oral polio and diphtheria-tetanus-pertussis vaccines administered at 2 months of age.(16) **Sexual Exposure to an HBsAg-positive Person** All susceptible persons whose sex partners have acute hepatitis B infection should receive a single dose of Hepatitis B Immune Globulin (Human) (0.06 mL/kg) and should begin the hepatitis B vaccine series if prophylaxis can be started within 14 days of the last sexual contact or if sexual contact with the infected person will continue (see Table 2 below). Administering the vaccine with Hepatitis B Immune Globulin may improve the efficacy of postexposure treatment. The vaccine has the added advantage of conferring long-lasting protection.(9) 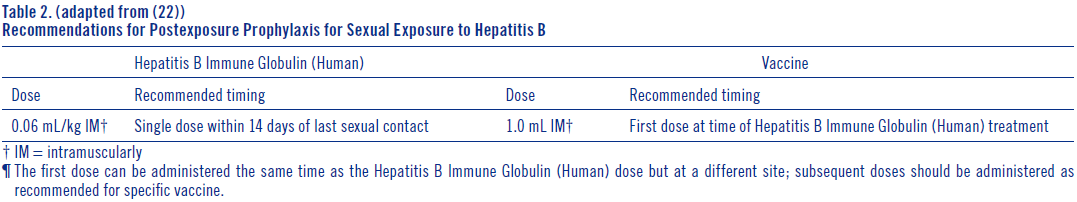 **Household Exposure to Persons with Acute HBV Infection** Prophylactic treatment with a 0.5 mL dose of Hepatitis B Immune Globulin (Human) and hepatitis B vaccine is indicated for infants <12 months of age who have been exposed to a primary care-giver who has acute hepatitis B. Prophylaxis for other household contacts of persons with acute HBV infection is not indicated unless they have had identifiable blood exposure to the index patient, such as by sharing toothbrushes or razors. Such exposures should be treated like sexual exposures. If the index patient becomes an HBV carrier, all household contacts should receive hepatitis B vaccine.(9) Hepatitis B Immune Globulin (Human) may be administered at the same time (but at a different site), or up to 1 month preceding Hepatitis B Vaccination without impairing the active immune response from Hepatitis B Vaccination.(17) Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Administer intramuscularly. Do not inject intravenously. Hyper **HEP B** ® is supplied in a syringe with an attached needle guard for your protection and convenience, as well as in vials. Please follow instructions below for proper use of syringe and needle guard. **Directions for Syringe Usage** 1. Remove the prefilled syringe from the package. Lift syringe by barrel, not by plunger. 2. Twist the plunger rod clockwise until the threads are seated. Do not use if the syringe is prematurely engaged. 3. With the needle shield secured on the syringe tip, push the plunger rod forward a few millimeters to break any friction seal between the stopper and the glass syringe barrel. 4. Remove the needle shield and expel air bubbles. \[Do not remove the needle shield to prepare the product for administration until immediately prior to the anticipated injection time.\] 5. Proceed with hypodermic needle puncture. 6. Aspirate prior to injection to confirm that the needle is not in a vein or artery. 7. Inject the medication. 8. Keeping your hands behind the needle, grasp the guard with free hand and slide forward toward needle until it is completely covered and guard clicks into place. If audible click is not heard, guard may not be completely activated. (See Diagrams A and B) 9. Place entire prefilled glass syringe with guard activated into an approved sharps container for proper disposal. (See Diagram C) 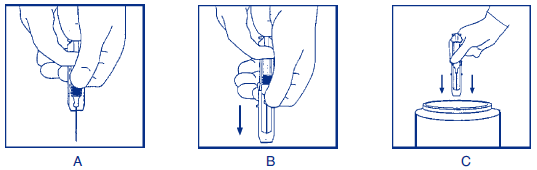 A number of factors could reduce the efficacy of this product or even result in an ill effect following its use. These include improper storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration and biological differences in individual patients. Because of these factors, it is important that this product be stored properly and that the directions be followed carefully during use. * * * **REFERENCES** 1. Recommendations of the Immunization Practices Advisory Committee (ACIP): Hepatitis B Virus: A Comprehensive Strategy for Eliminating Transmission in the United States Through Universal Childhood Vaccination. Appendix A: Postexposure Prophylaxis for Hepatitis B. _MMWR_ 40(RR-13):21–25, 1991. 2. Recommendation of the Immunization Practices Advisory Committee (ACIP): Recommendations for protection against viral hepatitis. _MMWR_ 34(22):313–35, 1985. 3. Szmuness W, Stevens CE, Olesko WR, et al: Passive-active immunisation against hepatitis B: immunogenicity studies in adult Americans. _Lancet_ 1:575–77, 1981. 4. Recommendations of the Immunization Practices Advisory Committee (ACIP): Update on Adult Immunization. Table 9. Recommendations for postexposure prophylaxis for percutaneous or permucosal exposure to hepatitis B, United States. _MMWR_ 40(RR-12):70, 1991. 5. Recommendations of the Immunization Practices Advisory Committee (ACIP): Update on Adult Immunization. Table 10. Recommendations for postexposure prophylaxis for perinatal and sexual exposure to hepatitis B, United States. _MMWR_ 40(RR-12):71, 1991.
INTRAMUSCULAR
Medical Information
**INDICATIONS AND USAGE** Recommendations on post-exposure prophylaxis are based on available efficacy data and on the likelihood of future HBV exposure for the person requiring treatment. In all exposures, a regimen combining Hepatitis B Immune Globulin (Human) with hepatitis B vaccine will provide both short- and long-term protection, will be less costly than the two-dose Hepatitis B Immune Globulin (Human) treatment alone, and is the treatment of choice.(9) Hyper **HEP B** is indicated for post-exposure prophylaxis in the following situations: **Acute Exposure to Blood Containing HBsAg** After either parenteral exposure, e.g., by accidental “needlestick” or direct mucous membrane contact (accidental splash), or oral ingestion (pipetting accident) involving HBsAg-positive materials such as blood, plasma or serum. For inadvertent percutaneous exposure, a regimen of two doses of Hepatitis B Immune Globulin (Human), one given after exposure and one a month later, is about 75% effective in preventing hepatitis B in this setting. **Perinatal Exposure of Infants Born to HBsAg-positive Mothers** Infants born to HBsAg-positive mothers are at risk of being infected with hepatitis B virus and becoming chronic carriers.(6,9–11) This risk is especially great if the mother is HBeAg-positive.(13–15) For an infant with perinatal exposure to an HBsAg-positive and HBeAg-positive mother, a regimen combining one dose of Hepatitis B Immune Globulin (Human) at birth with the hepatitis B vaccine series started soon after birth is 85%–95% effective in preventing development of the HBV carrier state.(9,15) Regimens involving either multiple doses of Hepatitis B Immune Globulin (Human) alone or the vaccine series alone have 70%–90% efficacy, while a single dose of Hepatitis B Immune Globulin (Human) alone has only 50% efficacy.(9,16) **Sexual Exposure to an HBsAg-positive Person** Sex partners of HBsAg-positive persons are at increased risk of acquiring HBV infection. For sexual exposure to a person with acute hepatitis B, a single dose of Hepatitis B Immune Globulin (Human) is 75% effective if administered within 2 weeks of last sexual exposure.(9) **Household Exposure to Persons with Acute HBV Infection** Since infants have close contact with primary care-givers and they have a higher risk of becoming HBV carriers after acute HBV infection, prophylaxis of an infant less than 12 months of age with Hepatitis B Immune Globulin (Human) and hepatitis B vaccine is indicated if the mother or primary care-giver has acute HBV infection.(9) Administration of Hepatitis B Immune Globulin (Human) either preceding or concomitant with the commencement of active immunization with Hepatitis B Vaccine provides for more rapid achievement of protective levels of hepatitis B antibody, than when the vaccine alone is administered.(17) Rapid achievement of protective levels of antibody to hepatitis B virus may be desirable in certain clinical situations, as in cases of accidental inoculations with contaminated medical instruments.(17) Administration of Hepatitis B Immune Globulin (Human) either 1 month preceding or at the time of commencement of a program of active vaccination with Hepatitis B Vaccine has been shown not to interfere with the active immune response to the vaccine.(17) * * * **REFERENCES** 1. Jhaveri R, Rosenfeld W, Salazar JD, et al: High titer multiple dose therapy with HBIG in newborn infants of HBsAg positive mothers. _J Pediatr_ 97(2):305–8, 1980. 2. Recommendations of the Immunization Practices Advisory Committee (ACIP): Hepatitis B Virus: A Comprehensive Strategy for Eliminating Transmission in the United States Through Universal Childhood Vaccination. Appendix A: Postexposure Prophylaxis for Hepatitis B. _MMWR_ 40(RR-13):21–25, 1991. 3. Stevens CE, Beasley RP, Tsui J, et al: Vertical transmission of hepatitis B antigen in Taiwan. _N Engl J Med_ 292(15):771–4, 1975. 4. Shiraki K, Yoshihara N, Kawana T, et al: Hepatitis B surface antigen and chronic hepatitis in infants born to asymptomatic carrier mothers. _Am J Dis Child_ 131(6):644–7, 1977. 5. Okada K, Kamiyama I, Inomata M, et al: e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. _N Engl J Med_ 294(14):746–9, 1976. 6. Beasley RP, Trepo C, Stevens CE, et al: The e antigen and vertical transmission of hepatitis B surface antigen. _Am J Epidemiol_ 105(2):94–8, 1977. 7. Beasley RP, Hwang LY, Lee GCY, et al: Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. _Lancet_ 2(8359): 1099–102, 1983. 8. Recommendation of the Immunization Practices Advisory Committee (ACIP): Recommendations for protection against viral hepatitis. _MMWR_ 34(22):313–35, 1985. 9. Szmuness W, Stevens CE, Olesko WR, et al: Passive-active immunisation against hepatitis B: immunogenicity studies in adult Americans. _Lancet_ 1:575–77, 1981.
**CONTRAINDICATIONS** None known.
J06BB04
hepatitis B immunoglobulin
Manufacturer Information
GRIFOLS ASIA PACIFIC PTE. LTD.
Grifols Therapeutics LLC
Active Ingredients
Documents
Package Inserts
HyperHEP B PI.pdf
Approved: March 17, 2023
