Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**2.2 DOSAGE AND ADMINISTRATION** Substitution by any other biological medicinal product requires the consent of the prescribing physician. The safety and efficacy of alternating or switching between Actemra and products that are biosimilar but not deemed interchangeable to Actemra has not been established. Therefore, the benefit/risk of alternating or switching need to be carefully considered. For adult patients with RA, tocilizumab may be administered as an IV infusion or a SC injection. For adult patients with COVID-19, tocilizumab is administered as an IV infusion. For patients with pJIA, tocilizumab is administered as an IV infusion. For patients with sJIA, tocilizumab is administered as an IV infusion. _Intravenous Administration_ Actemra IV formulation is not intended for subcutaneous administration. Actemra IV formulation should be diluted by a healthcare professional with sterile 0.9% w/v sodium chloride solution using aseptic technique ( _see section 4.2 Special Instructions for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Actemra is recommended for IV infusion over 1 hour. _Subcutaneous Administration_ Actemra SC formulation is not intended for intravenous administration. Actemra SC formulation is administered with a single-use PFS+NSD. The first injection should be performed under the supervision of a qualified health care professional. A patient can self-inject ACTEMRA only if the physician determines that it is appropriate and the patient agrees to medical follow-up as necessary and has been trained in proper injection technique. The recommended injection sites (abdomen, thigh and upper arm) should be rotated and injections should never be given into moles, scars, or areas where the skin is tender, bruised, red, hard, or not intact. Patients who transition from Actemra IV therapy to SC administration should administer the first SC dose at the time of the next scheduled IV dose under the supervision of a qualified health care professional. Assess suitability of patient or parent/guardian for SC home administration and instruct the patient or parent/guardian to inform a healthcare professional before administering the next dose, if any symptoms of allergic reaction are experienced. Patients should seek immediate medical attention if they develop symptoms of serious allergic reactions ( _see section 2.4.1 Warnings and Precautions, General and 2.6 Undesirable Effects_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Rheumatoid Arthritis (RA) \[IV and SC formulations\]** _Intravenous Dosing Regimen:_ The recommended dose of Actemra for adult patients is 8 mg/kg, but no lower than 480 mg, given once every four weeks. Doses above 1.2 g have not been evaluated in clinical studies. Actemra should be diluted to 100 ml by a healthcare professional with sterile 0.9% w/v sodium chloride solution using aseptic technique ( _see section 4.2 Special Instructions for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Actemra is recommended for IV infusion over 1 hour. For individuals whose body weight is more than 100 kilograms (kg), doses exceeding 800 mg per infusion are not recommended ( _see Section 3.2 Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Subcutaneous Dosing Regimen:_ The recommended dose of Actemra for adult patients is 162 mg given once every week as a subcutaneous injection. Actemra can be used alone or in combination with MTX and/or other DMARDs. _Dose Modification Recommendations for RA_ (See Section 2.4.1 Warnings and Precautions, General – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - Liver enzyme abnormalities 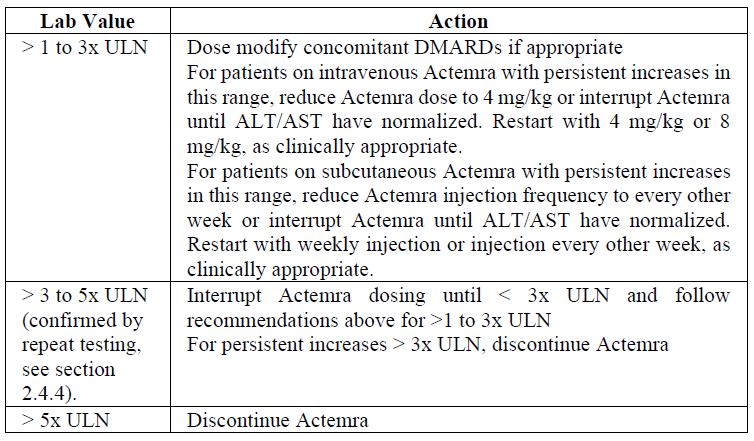 - Low absolute neutrophil count (ANC) 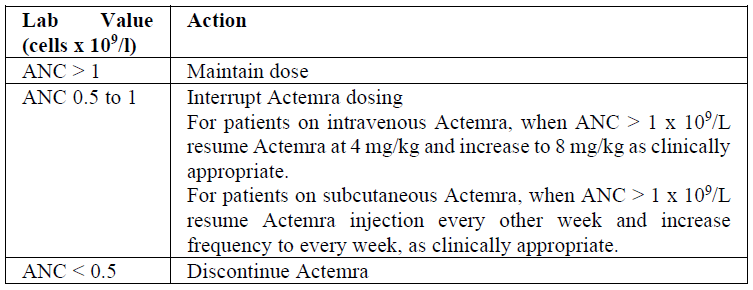 - Low platelet count 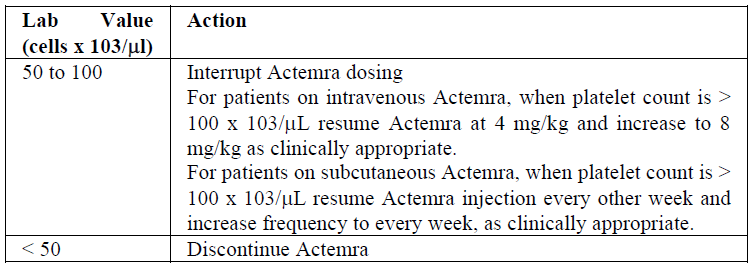 **COVID-19 \[IV formulation only\]** The recommended dose of tocilizumab for treatment of adult patients with COVID-19 is a single 60-minute infusion of 8 mg/kg. If clinical signs or symptoms worsen or do not improve after the first dose, one additional infusion of tocilizumab 8 mg/kg may be administered at least 8 hours after the initial infusion. Doses exceeding 800 mg per infusion are not recommended in patients with COVID-19. **Cytokine Release Syndrome (CRS) (adults and paediatrics) \[IV formulation only\]** The recommended dose of Actemra for treatment of patients with CRS given as a 60-minute intravenous infusion is: - 12 mg/kg for patients below 30 kg, - 8 mg/kg for patients ≥ 30 kg, Actemra can be used alone or in combination with corticosteroids. If no clinical improvement in the signs and symptoms of CRS occurs after the first dose, up to 3 additional doses of Actemra may be administered. The interval between consecutive doses should be at least 8 hours. Doses exceeding 800 mg per infusion are not recommended in CRS patients. Patients with severe or life-threatening CRS frequently have cytopenias or elevated ALT or AST due to the underlying malignancy, preceding lymphodepleting chemotherapy or the CRS. **Polyarticular Juvenile Idiopathic Arthritis (pJIA) \[IV formulation only\]** The recommended dose of Actemra for patients with pJIA is: - 10 mg/kg for patients below 30 kg, - 8 mg/kg for patients ≥ 30 kg, given once every four weeks as an IV infusion. A change in dose should only be based on a consistent change in the patient’s body weight over time. Actemra can be used alone or in combination with MTX. **Systemic juvenile idiopathic arthritis (sJIA) \[IV formulation only\]** The recommended dose of Actemra for patients with sJIA is: - 12 mg/kg for patients below 30 kg, - 8 mg/kg for patients ≥ 30 kg, given once every two weeks as an IV infusion. A change in dose should only be based on a consistent change in the patient’s body weight over time. Actemra can be used alone or in combination with MTX. Actemra should be diluted by a healthcare professional with sterile 0.9% w/v sodium chloride solution using aseptic technique ( _see section 4.2 Special Instructions for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Actemra is recommended for IV infusion over 1 hour. _Dose Modification Recommendations for pJIA and sJIA:_ Dose reduction of Actemra has not been studied in the pJIA or sJIA population. Dose interruptions of Actemra for laboratory abnormalities are recommended in patients with pJIA or sJIA and are similar to what is outlined above for patients with RA ( _see Section 2.4.1 Warnings and Precautions, General_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If appropriate, concomitant methotrexate and/or other medications should be dose modified or stopped and Actemra dosing interrupted until the clinical situation has been evaluated. In pJIA or sJIA the decision to discontinue Actemra for a laboratory abnormality should be based upon the medical assessment of the individual patient. **2.2.1 Special Dosage Instructions** _Pediatric use:_ The safety and efficacy of Actemra intravenous formulation in paediatric patients below the age of 2 years old have not been established. The safety and efficacy of Actemra subcutaneous formulation in children from birth to less than 18 years have not been established. No data are available. _Geriatric use:_ No dose adjustment is required in elderly patients > 65 years of age. _Renal impairment:_ No dose adjustment is required in patients with mild or moderate renal impairment (see section 3.2.3 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Actemra has not been studied in patients with severe renal impairment. _Hepatic impairment:_ The safety and efficacy of Actemra has not been studied in patients with hepatic impairment (see section 2.4.1 Warnings and Precautions, General – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
SUBCUTANEOUS
Medical Information
**2.1 THERAPEUTIC INDICATION(S)** **Rheumatoid Arthritis \[IV and SC formulations\]** Actemra, in combination with methotrexate (MTX) or other disease-modifying anti-rheumatic drugs (DMARDs), is indicated for: - the treatment of severe, active and progressive rheumatoid arthritis (RA) in adults not previously treated with MTX \[IV formulation only\] - the treatment of moderate to severe active rheumatoid arthritis (RA) in adult patients who have either responded inadequately to, or who were intolerant to, previous therapy with one or more disease-modifying anti-rheumatic drugs (DMARDs) or tumour necrosis factor (TNF) antagonists \[IV and SC formulations\] In these patients, Actemra can be used alone in case of intolerance to MTX or where continued treatment with MTX is inappropriate. Tocilizumab has been shown to reduce the rate of progression of joint damage as measured by X-ray and to improve physical function when given in combination with methotrexate. **Coronavirus disease 2019 (COVID-19) \[IV formulation only\]** Tocilizumab is indicated for the treatment of coronavirus disease 2019 (COVID-19) in hospitalized adults who are receiving systemic corticosteroids and require supplemental oxygen or mechanical ventilation. **Polyarticular Juvenile Idiopathic Arthritis (pJIA) \[IV formulation only\]** Actemra is indicated in combination with methotrexate (MTX) for the treatment of active polyarticular juvenile idiopathic arthritis (pJIA) in patients 2 years of age and older, who have responded inadequately to previous therapy with MTX. Actemra can be given alone in case of intolerance to MTX or where continued treatment with MTX is inappropriate. **Systemic Juvenile Idiopathic Arthritis (sJIA) \[IV formulation only\]** Actemra is indicated for the treatment of active systemic juvenile idiopathic arthritis in patients 2 years of age and older, who have responded inadequately to previous therapy with NSAIDS and systemic corticosteroids. Actemra can be given alone or in combination with MTX. **Chimeric Antigen Receptor (CAR) T cell-induced severe or life-threatening Cytokine Release Syndrome (CRS) \[IV formulation only\]** Actemra is indicated for the treatment of chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome (CRS) in adults and paediatric patients 2 years of age and older.
**2.3 CONTRAINDICATIONS** Actemra is contraindicated in patients with a known hypersensitivity to the active substance or to any of the excipients. Active, severe infections.
L04AC07
tocilizumab
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Vetter Pharma-Fertigung GmbH & Co KG
Active Ingredients
Documents
Package Inserts
Actemra Concentrate for Solution for Infusion PI.pdf
Approved: May 5, 2021
