Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**2 DOSAGE AND ADMINISTRATION** General Dosing Recommendations: - REYATAZ Capsules must be taken with food. - Do not open the capsules. - The recommended oral dosage of REYATAZ depends on the treatment history of the patient and the use of other coadministered drugs \[see _Drug Interactions (7.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. When coadministered with H2-receptor antagonists or proton-pump inhibitors, dose separation may be required \[see _Dosage and Administration (2.1)_\]. - When coadministered with didanosine buffered or enteric-coated formulations, REYATAZ should be given (with food) 2 hours before or 1 hour after didanosine. - REYATAZ without ritonavir is not recommended for treatment-experienced adults or pediatric patients with prior virologic failure \[see _Clinical Studies (13)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. - Efficacy and safety of REYATAZ with ritonavir in doses greater than 100 mg once daily have not been established. The use of higher ritonavir doses might alter the safety profile of atazanavir (cardiac effects, hyperbilirubinemia) and, therefore, is not recommended. Prescribers should consult the complete prescribing information for ritonavir when using this agent. **2.1 Recommended Adult Dosage** Table 1 summarizes the recommended REYATAZ dosing regimen in adults. All REYATAZ dosing regimens are to be administered as a single dose with food.  \[For these drugs and other antiretroviral agents for which dosing modification may be appropriate, see _Drug Interactions (7)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.\] **2.2 Recommended Pediatric Dosage** The recommended daily dosage of REYATAZ capsules for pediatric patients (6 to less than 18 years of age) is based on body weight and should not exceed the recommended adult dosage. REYATAZ capsules must be taken with food. The data are insufficient to recommend dosing of REYATAZ capsules for any of the following: (1) patients less than 6 years of age, (2) without ritonavir in any pediatric patient less than 13 years of age, and (3) patients less than 40 kg receiving concomitant tenofovir, H2-receptor antagonists, or proton-pump inhibitors. The recommended dosage of REYATAZ with ritonavir in pediatric patients at least 6 years of age is shown in Table 2. 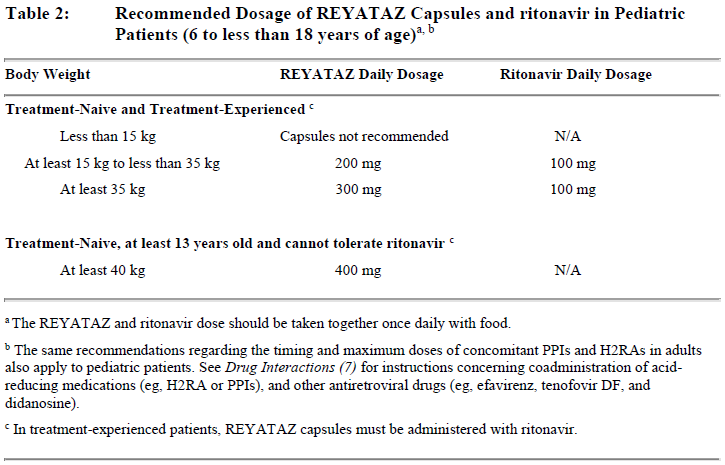 For treatment-naive patients at least 13 years of age and at least 40 kg, who are unable to tolerate ritonavir, the recommended dose is REYATAZ 400 mg (without ritonavir) once daily with food. For patients at least 13 years of age and at least 40 kg receiving concomitant tenofovir, H2-receptor antagonists, or proton-pump inhibitors, REYATAZ should not be administered without ritonavir. **2.3 Pregnancy** _Dosing During Pregnancy and the Postpartum Period:_ - REYATAZ should not be administered without ritonavir. - REYATAZ should only be administered to pregnant women with HIV-1 strains susceptible to atazanavir. - For pregnant patients, no dose adjustment is required for REYATAZ with the following exceptions: - For treatment-experienced pregnant women during the second or third trimester, when REYATAZ is coadministered with either an H2-receptor antagonist **or** tenofovir DF, REYATAZ 400 mg with ritonavir 100 mg once daily is recommended. There are insufficient data to recommend a REYATAZ dose for use with both an H2-receptor antagonist _and_ tenofovir DF in treatment-experienced pregnant women. - During the second and third trimesters of pregnancy, REYATAZ 300 mg with ritonavir 100 mg may not provide sufficient exposure to atazanavir, especially when the activity of atazanavir or the whole regimen may be compromised due to drug resistance. Since there are limited data available and due to inter-patient variability during pregnancy, Therapeutic Drug Monitoring (TDM) may be considered to ensure adequate exposure. - No dose adjustment is required for postpartum patients. However, patients should be closely monitored for adverse events because atazanavir exposures could be higher during the first 2 months after delivery. \[See _Use in Specific Populations (8.1)_ and _Clinical Pharmacology (11.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.\] **2.4 Renal Impairment** For patients with renal impairment, including those with severe renal impairment who are not managed with hemodialysis, no dose adjustment is required for REYATAZ. Treatment-naive patients with end stage renal disease managed with hemodialysis should receive REYATAZ 300 mg with ritonavir 100 mg. REYATAZ without ritonavir should not be administered to treatment-naive patients managed with hemodialysis. REYATAZ should not be administered to HIV-treatment-experienced patients with end stage renal disease managed with hemodialysis. \[See _Use in Specific Populations (8.6)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.\] **2.5 Hepatic Impairment** REYATAZ should be used with caution in patients with mild-to-moderate hepatic impairment. For patients with moderate hepatic impairment (Child-Pugh Class B) who have not experienced prior virologic failure, a dose reduction to 300 mg once daily should be considered. REYATAZ should not be used in patients with severe hepatic impairment (Child-Pugh Class C). REYATAZ/ritonavir has not been studied in subjects with hepatic impairment and is not recommended. \[See _Warnings and Precautions (5.5)_ and _Use in Specific Populations (8.7)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.\]
ORAL
Medical Information
**1 INDICATIONS AND USAGE** REYATAZ \[atazanavir (as sulfate)\] is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. This indication is based on analyses of plasma HIV-1 RNA levels and CD4+ cell counts from controlled studies of 96 weeks duration in antiretroviral-naive and 48 weeks duration in antiretroviral-treatment-experienced adult patients, and limited data from children aged 6 to less than 18 years. The following points should be considered when initiating therapy with REYATAZ: - In Study AI424-045 REYATAZ/ritonavir and lopinavir/ritonavir were similar for the primary efficacy outcome measure of time-averaged difference in change from baseline in HIV RNA level. This study was not large enough to reach a definitive conclusion that REYATAZ/ritonavir and lopinavir/ritonavir are equivalent on the secondary efficacy outcome measure of proportions below the HIV RNA lower limit of detection \[see _Clinical Studies (13.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. - The number of baseline primary protease inhibitor mutations affects the virologic response to REYATAZ/ritonavir \[see _Clinical Pharmacology (11.4)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
**4 CONTRAINDICATIONS** REYATAZ is contraindicated: - in patients with previously demonstrated clinically significant hypersensitivity (eg, Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of this product. - when coadministered with drugs that are highly dependent on CYP3A or UGT1A1 for clearance, and for which elevated plasma concentrations are associated with serious and/or life-threatening events. - when coadministered with drugs that strongly induce CYP3A and may lead to lower exposure and loss of efficacy of REYATAZ. These and other contraindicated drugs are listed in Table 3. 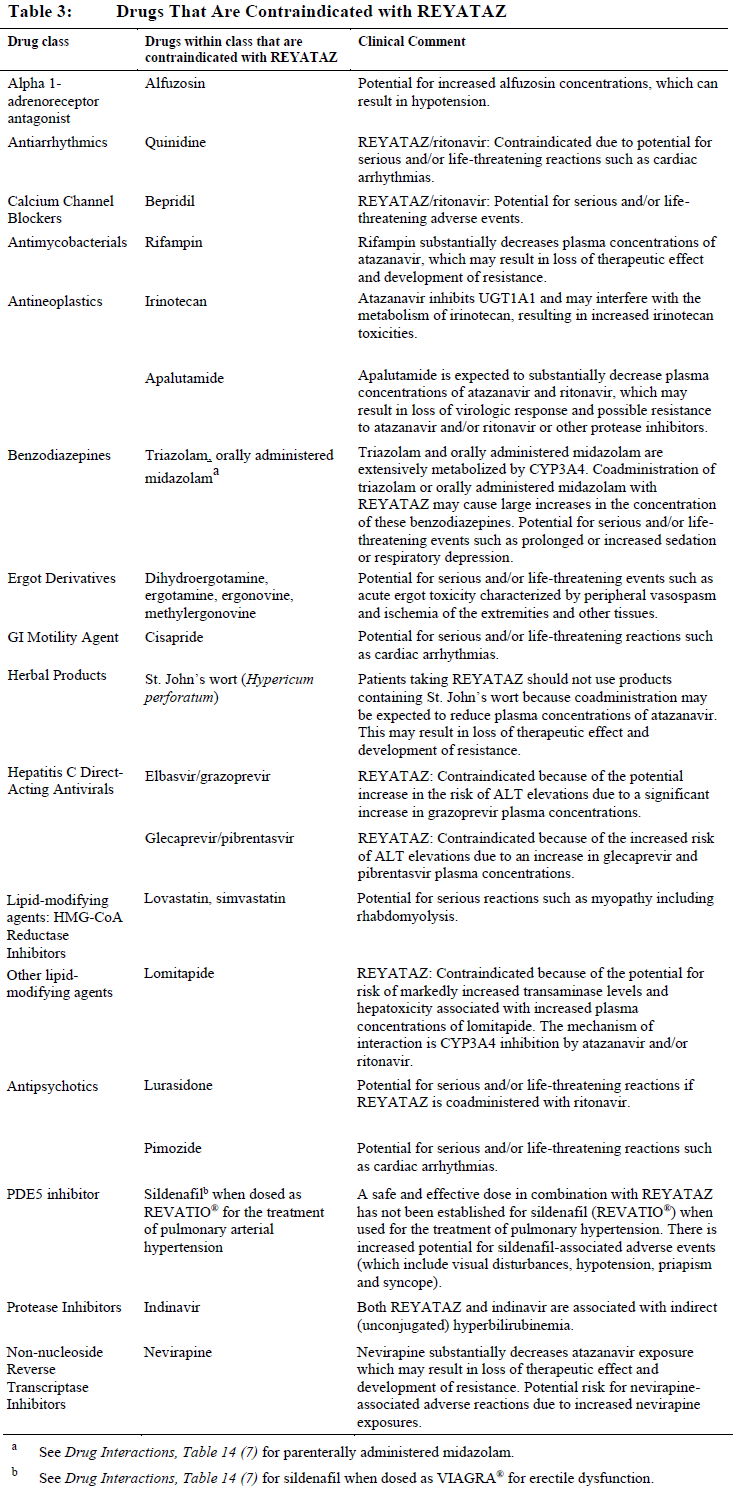
J05AE08
atazanavir
Manufacturer Information
BRISTOL-MYERS SQUIBB (SINGAPORE) PTE. LTD.
AstraZeneca Pharmaceuticals LP
AndersonBrecon Incorporated (Primary and Secondary Packager)
Patheon Inc.
Active Ingredients
Documents
Package Inserts
Reyataz Capsule PI.pdf
Approved: June 20, 2023
