Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SUSPENSION, EXTENDED RELEASE
**Dosage and Administration** REKAMBYS® should always be co-administered with cabotegravir injection. Therefore, the cabotegravir injection prescribing information should be consulted. Prior to starting REKAMBYS®, the healthcare professional should carefully select patients who agree to the required injection schedule and counsel patients about the importance of adherence to scheduled dosing visits to help maintain viral suppression and reduce the risk of viral rebound and potential development of resistance associated with missed doses. **Method of administration** For gluteal intramuscular (IM) injection use only. **Do not inject intravenously**. REKAMBYS® should be administered by a healthcare professional. When administering REKAMBYS®, the healthcare professional should take into consideration the body mass index (BMI) of the patient to ensure that the needle length is sufficient to reach the gluteus muscle. REKAMBYS® and cabotegravir injections should be administered at separate gluteal injection sites during the same visit. **Posology** _**Oral lead-in**_ EDURANT is recommended for approximately 1 month (at least 28 days) in virologically suppressed patients prior to the initiation of REKAMBYS® to assess tolerability to rilpivirine. One EDURANT 25 mg tablet should be taken once daily with a meal and should be administered with one cabotegravir 30 mg tablet once daily. The EDURANT prescribing information should be consulted. **Every 1 month dosing** _**Initiation injection (3 mL dose)**_ On the final day of oral lead-in, the recommended initiation dose of REKAMBYS® in adults is a single 3 mL (900 mg) intramuscular injection. _**Continuation injections (2 mL dose)**_ After the initiation injection, the recommended continuation injection dose of REKAMBYS® in adults is a single 2 mL (600 mg) monthly intramuscular injection. Patients may be given injections up to 7 days before or after the date of the monthly 2 mL injection schedule. 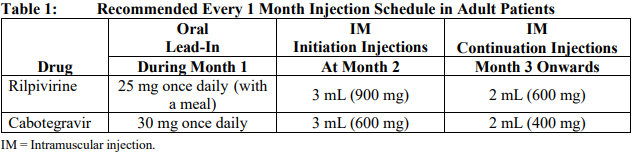 **Every 2 months dosing** _**Initiation injections (3 mL dose)**_ On the final day of oral lead-in, the recommended initial REKAMBYS® injection dose in adults is a single 3 mL (900 mg) intramuscular injection (Month 2). One month later (Month 3), a second 3 mL (900 mg) intramuscular injection should be administered. Patients may be given the second 3 mL (900 mg) injection up to 7 days before or after the scheduled dosing date. _**Continuation injections (3 mL dose)**_ After the second initiation injection, the recommended REKAMBYS® continuation injection dose in adults is a single 3 mL (900 mg) intramuscular injection administered every 2 months beginning at Month 5. Patients may be given injections up to 7 days before or after the date of the every 2 months 3 mL injection schedule. 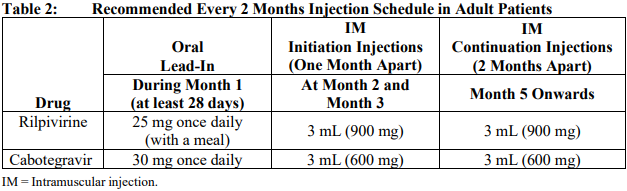 **Change in dosing frequency** _**Dosing recommendation when switching from monthly to every 2 months injections**_ Patients switching from a monthly continuation injection schedule to an every 2 months continuation injection schedule should receive a single 3 mL (900 mg) intramuscular injection of REKAMBYS® one month after the last 2 mL (600 mg) continuation injection dose and then 3 mL (900 mg) every 2 months thereafter. _**Dosing recommendations when switching from every 2 months to monthly injections**_ Patients switching from an every 2 months continuation injection schedule to a monthly continuation injection schedule should receive a single 2 mL (600 mg) intramuscular injection of REKAMBYS® two months after the last 3 mL (900 mg) REKAMBYS® continuation injection dose and then 2 mL (600 mg) monthly thereafter. **Missed dose(s)** Adherence to the injection schedule is strongly recommended. Patients who miss an injection visit should be clinically reassessed to ensure resumption of therapy is appropriate. See Table 3 and 4 for dosing recommendations after a missed injection. _**Missed every 1 month injection dose**_ If a delay of more than 7 days from a scheduled injection visit cannot be avoided, oral therapy (EDURANT \[25 mg\] and cabotegravir tablets \[30 mg\] once daily) may be used to replace up to 2 consecutive monthly injection visits. The first dose of oral therapy should be taken 1 month (±7 days) after the last injection dose of REKAMBYS® and cabotegravir. Injection dosing should be resumed on the day oral dosing completes, as recommended in Table 3.  _**Missed every 2 months injection dose**_ If a delay of more than 7 days from a scheduled injection visit cannot be avoided, oral therapy (EDURANT \[25 mg\] and cabotegravir tablets \[30 mg\] once daily) may be used to replace one “every 2 months” injection visit. The first dose of oral therapy should be taken 2 months (±7 days) after the last injection doses of REKAMBYS® and cabotegravir. Injection dosing should be resumed on the day oral dosing completes, as recommended in Table 4. 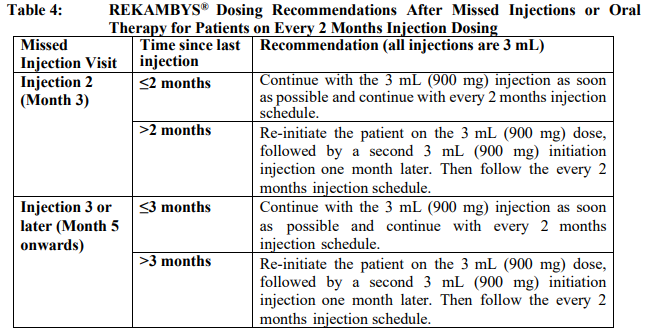 **Transition from REKAMBYS® to another HIV treatment** A fully suppressive antiretroviral regimen should be initiated no later than one month after the final injection of REKAMBYS® when dosed monthly and no later than 2 months after the final injection of REKAMBYS® when dosed every 2 months. If virologic failure is suspected, an alternative regimen should be initiated as soon as possible. **Special populations** _**Pediatrics (17 years of age and younger)**_ The safety and efficacy of REKAMBYS® have not been established in pediatric patients. Treatment with REKAMBYS® is not recommended in this population. _**Elderly (65 years of age and older)**_ There is limited information regarding the use of REKAMBYS® in patients >65 years of age. No dose adjustment of REKAMBYS® is required in elderly patients (see _Pharmacokinetic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Renal impairment**_ No dose adjustment of REKAMBYS® is required in patients with mild and moderate renal impairment. In patients with severe renal impairment or end stage renal disease, the combination of REKAMBYS® with a strong CYP3A inhibitor should only be used if the benefit outweighs the risk. Subjects with estimated creatinine clearance < 50mL/min/1.73m2 were not included in the Phase 3 studies. No data are available in subjects receiving dialysis although differences in pharmacokinetics are not expected in this population (see _Pharmacokinetic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Hepatic impairment**_ No dose adjustment of REKAMBYS® is required in patients with mild or moderate hepatic impairment (Child-Pugh score A or B) but caution is advised in patients with moderate hepatic impairment. REKAMBYS® has not been studied in patients with severe hepatic impairment (Child-Pugh score C) therefore REKAMBYS® is not recommended in these patients (see _Pharmacokinetic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAMUSCULAR
Medical Information
**Indications** REKAMBYS® is indicated, in combination with cabotegravir injection, for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with agents of the NNRTI and INI class (see _Pharmacological Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**Contraindications** Hypersensitivity to rilpivirine or to any of the excipients. REKAMBYS® should not be co-administered with the following medicinal products, as significant decreases in rilpivirine plasma concentrations may occur (due to CYP3A enzyme induction), which may result in loss of therapeutic effect of REKAMBYS® (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): - the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, phenytoin - the antimycobacterials rifabutin, rifampicin, rifapentine - the glucocorticoid systemic dexamethasone, except as a single dose treatment - St John’s wort ( _Hypericum perforatum_) When using oral rilpivirine, proton pump inhibitors are also contraindicated. The prescribing information for cabotegravir should also be consulted.
J05AG05
rilpivirine
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Cilag AG
Active Ingredients
Documents
Package Inserts
Rekambys IFU (2mL).pdf
Approved: February 10, 2023
