Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**DOSAGE AND ADMINISTRATION** AZACTAM may be administered intravenously or by intramuscular injection. Dosage and route of administration should be determined by susceptibility of the causative organisms, severity and site of infection, and the condition of the patient. 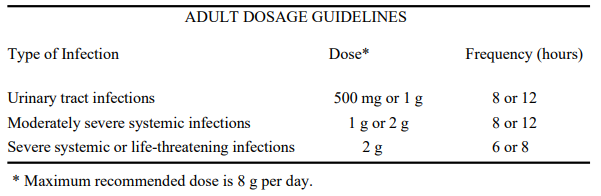 A single dose of 1 g of AZACTAM administered intramuscularly is effective in the treatment of acute uncomplicated gonorrhea and acute uncomplicated cystitis. The intravenous route is recommended for patients requiring single doses greater than 1 g or those with bacterial septicemia, localized parenchymal abscess (e.g., intra-abdominal abscess), peritonitis or other severe systemic or life-threatening infections. Because of the serious nature of infections due to _Pseudomonas aeruginosa_, dosage of 2 g every six or eight hours is recommended, at least upon initiation of therapy, in systemic infections caused by this organism. **Pediatric** The usual dosage for patients older than one week is 30 mg/kg every six to eight hours. For severe infections in patients two years or older, 50 mg/kg every six to eight hours is recommended. The recommended dose for all patients in the treatment of infections due to _P. aeruginosa_ is 50 mg/kg every six to eight hours. The maximum daily pediatric dose should not exceed the maximum recommended dose for adults. **Renal Impairment** Prolonged serum levels of aztreonam may occur in patients with transient or persistent renal insufficiency. Therefore, after an initial usual dose, the dosage of AZACTAM should be halved in patients with estimated creatinine clearances between 10 and 30 mL/min/1.73 m2. When only the serum creatinine concentration is available, the following formula (based on sex, weight, and age of the patient) may be used to approximate the creatinine clearance (Clcr). The serum creatinine should represent a steady state of renal function.  In patients with severe renal failure (creatinine clearance less than 10 mL/min/1.73 m2 ), such as those supported by hemodialysis, the usual dose should be given initially. The maintenance dose should be one-fourth of the usual initial dose given at the usual fixed interval of 6, 8 or 12 hours. For serious or life-threatening infections, in addition to the maintenance doses, one-eighth of the initial dose should be given after each hemodialysis session. **Dosage in the Elderly** Renal status is a major determinant of dosage in the elderly; these patients in particular may have diminished renal function. Serum creatinine may not be an accurate determinant of renal status. Therefore, as with all antibiotics eliminated by the kidneys, estimates of creatinine clearance should be obtained, and appropriate dosage modifications made if necessary.
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**INDICATIONS** AZACTAM is indicated for the treatment of the infections listed below when caused by susceptible gram-negative microorganisms. Before initiating treatment with AZACTAM, appropriate specimens should be obtained for isolation of the causative organism(s) and for determination of susceptibility to aztreonam. Treatment may be started empirically before results of susceptibility testing are available. In infections where gram-positive or anaerobic pathogens are suspected or are shown to be present, AZACTAM must be used with another antibiotic to provide appropriate coverage. See **Concurrent Therapy.** **Urinary Tract Infections** (complicated and uncomplicated), including pyelonephritis and cystitis (initial and recurrent) and asymptomatic bacteriuria. **Lower Respiratory Tract Infections,** including pneumonia and bronchitis. In the treatment of acute pulmonary exacerbations in patients with cystic fibrosis, clinical improvement is usually noted. **Bacteremia/Septicemia** **Meningitis** caused by _Haemophilus influenzae_ and _Neisseria meningitidis._ **Bone and Joint Infections** **Skin and Skin-Structure Infections,** including those associated with post-operative wounds, ulcers and burns. **Intra-abdominal Infections,** including peritonitis. **Gynecologic Infections,** including pelvic inflammatory disease, endometritis and pelvic cellulitis. **Gonorrhea** (acute uncomplicated urogenital or anorectal infections due to beta-lactamase producing or non-producing strains of _N. gonorrhoeae_). AZACTAM is indicated for adjunctive therapy to surgery in the management of infections caused by susceptible organisms, including abscesses, infections complicating hollow viscus perforations, cutaneous infections, and infections of serous surfaces. AZACTAM is effective against most of the commonly encountered gram-negative aerobic pathogens seen in general surgery. **Concurrent Therapy** Concurrent initial therapy with other antimicrobial agents and AZACTAM is recommended before the causative organism(s) is known in seriously ill patients who are also at risk of having an infection due to gram-positive aerobic pathogens. If anaerobic organisms are also suspected as etiologic agents, therapy should be initiated using an anti-anaerobic agent concurrently with AZACTAM. Certain antibiotics (eg, cefoxitin, imipenem) may induce high levels of beta-lactamase _in vitro_ in some gram-negative aerobes such as _Enterobacter_ and _Pseudomonas_ species, resulting in antagonism to many beta-lactam antibiotics including aztreonam. These _in vitro_ findings suggest that such beta-lactamase-inducing antibiotics not be used concurrently with aztreonam. Following identification and susceptibility testing of the causative organism(s), appropriate antibiotic therapy should be continued. Some patients with serious infections due to _Pseudomonas_ may benefit from concurrent use of AZACTAM and an aminoglycoside because of synergistic action. These agents are also synergistic _in vitro_ against many strains of _Enterobacteriaceae_, and other gram negative aerobic bacilli. However, this enhanced activity is not predictable.
**CONTRAINDICATIONS** This preparation is contraindicated in patients with known hypersensitivity to aztreonam or any other component in the formulation.
J01DF01
aztreonam
Manufacturer Information
BRISTOL-MYERS SQUIBB (SINGAPORE) PTE. LTD.
CATALENT ANAGNI S.R.L.
Active Ingredients
Documents
Package Inserts
Azactam For Injection PI.pdf
Approved: July 22, 2020
