Regulatory Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
Therapeutic
Prescription Only
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** ACTILYSE should be given as early as possible after symptom onset. _Posology_ **1\. Acute myocardial infarction** 1. 90 minutes (accelerated) dose regimen for patients with acute myocardial infarction, in whom treatment can be started within 6 hours after symptom onset. In patients with a body weight ≥ 65 kg: - 15 mg as an intravenous bolus, immediately followed by - 50 mg as an intravenous infusion over the first 30 minutes, immediately followed by an intravenous infusion of - 35 mg over 60 minutes, until the maximum total dose of 100 mg. In patients with a body weight < 65 kg the total dose should be weight adjusted with - 15 mg as an intravenous bolus, immediately followed by - 0.75 mg/kg body weight as an intravenous infusion over the first 30 minutes (maximum 50 mg), immediately followed by an intravenous infusion of - 0.5 mg/kg over 60 minutes (up to a maximum of 35 mg). 2. 3 hours dose regimen for patients with acute myocardial infarction, in whom treatment can be started between 6 and 12 hours after symptom onset: In patients with a body weight ≥ 65 kg: - 10 mg as an intravenous bolus, immediately followed by - 50 mg as an intravenous infusion over the first hour, immediately followed by an intravenous infusion of - 40 mg over two hours, until the maximum total dose of 100 mg. In patients with a body weight < 65 kg: - 10 mg as an intravenous bolus, immediately followed by - an intravenous infusion over three hours up to a maximum total dose of 1.5 mg/kg body weight. Adjunctive therapy: Antithrombotic adjunctive therapy is recommended according to the current international guidelines for the management of patients with ST-elevation myocardial infarction. 2\. **Acute massive pulmonary embolism** In patients with a body weight ≥ 65 kg: A total dose of 100 mg should be administered in 2 hours. The most experience available is with the following dose regimen: - 10 mg as an intravenous bolus over 1 – 2 minutes, immediately followed by - 90 mg as an intravenous infusion over two hours until the total dose of 100mg In patients with a body weight < 65 kg: - 10 mg as an intravenous bolus over 1 – 2 minutes, immediately followed by - an intravenous infusion up to a maximum total dose of 1.5 mg/kg. Adjunctive therapy: After treatment with ACTILYSE heparin therapy should be initiated (or resumed) when aPTT values are less than twice the upper limit of normal. The infusion should be adjusted to maintain aPTT between 50 – 70 seconds (1.5 to 2.5 fold of the reference value). 3\. **Acute ischaemic stroke** The recommended total dose is 0.9 mg/kg body weight (maximum of 90 mg) infused starting with 10% of the total dose as an initial intravenous bolus, immediately followed by the remainder of the total dose infused intravenously over 60 minutes. Treatment should be initiated as early as possible within 4.5 hours of symptom onset, see section 4.4 Special warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The treatment effect is time-dependent; therefore earlier treatment increases the probability of a favourable outcome. Beyond 4.5 hours after onset of stroke symptoms there is a negative benefit risk ratio associated with Actilyse administration and so it should not be administered (see 5.1 Pharmacological properties – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 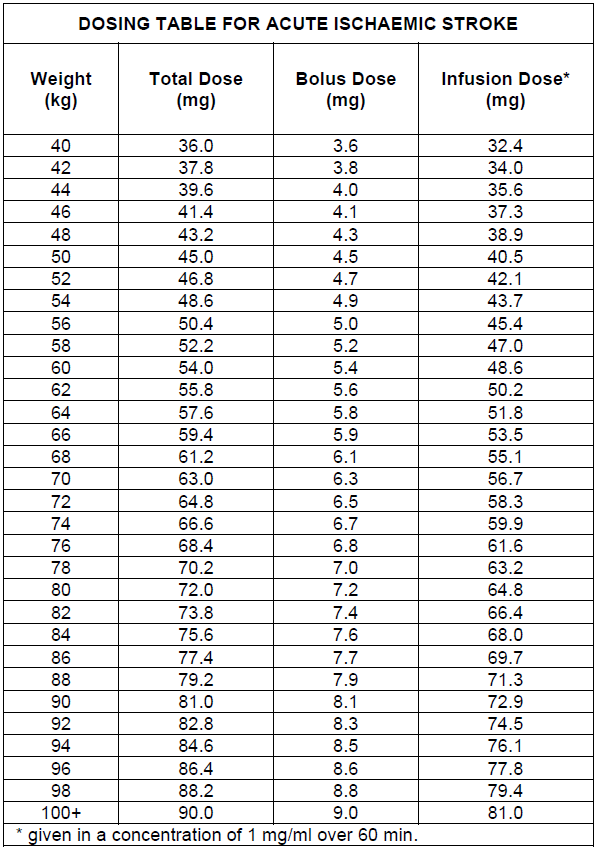 Adjunctive therapy: The safety and efficacy of this regimen with concomitant administration of heparin or platelet aggregation inhibitors such as acetylsalicylic acid during the first 24 hours after the symptom-onset has not been investigated sufficiently. Therefore, administration of intravenous heparin or platelet aggregation inhibitors such as acetylsalicylic acid should be avoided in the first 24 hours after treatment with ACTILYSE due to an increased haemorrhagic risk. If heparin is required for other indications (e.g. prevention of deep vein thrombosis) the dose should not exceed 10,000 international units per day, administered subcutaneously. _Method of_ administration The reconstituted solution should be administered intravenously and is for immediate use. Handling Instructions Under aseptic conditions the contents of an injection vial of ACTILYSE (50 mg) dry substance is dissolved with sterilised water for injection according to the following table to obtain a final concentration of 1 mg alteplase per ml.  For this purpose a transfer cannula is included with the pack-sizes of 50 mg. Instructions for reconstituting Actilyse 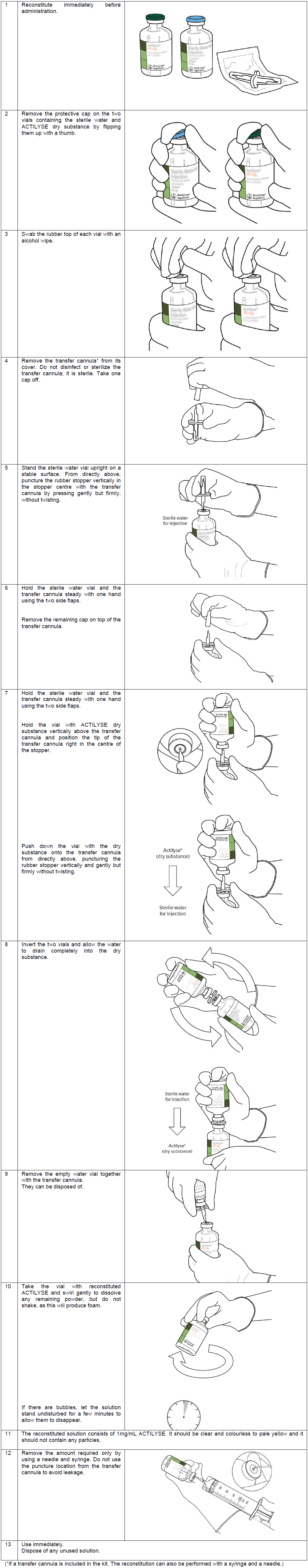 The 1mg/mL reconstituted solution may be diluted further with sterile sodium chloride 9 mg/mL (0.9% ) solution for Injection up to a minimal concentration of 0.2 mg/mL since the occurrence of turbidity of the reconstituted solution cannot be excluded. A further dilution of the 1mg/mL reconstituted solution with sterilised water for injections or in general, the use of carbohydrate infusion solutions, e.g. dextrose is not recommended due to increasing formation of turbidity of the reconstituted solution. ACTILYSE should not be mixed with other drugs, neither in the same infusion-vial nor the same venous line (not even with heparin). Traceability In order to improve traceability of biological medicinal products, the trade name and the batch number of the administered product should be clearly recorded in the patient file.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** 1. **Thrombolytic treatment in acute myocardial infarction.** - 90 minutes (accelerated) dose regimen (see section 4.2 Posology and administration): for patients in whom treatment can be started within 6 hours of symptom onset; - 3 hours dose regimen (see section 4.2 Posology and administration): for patients in whom treatment can be started between 6 – 12 hours after symptom onset. ACTILYSE has proven to reduce 30-day-mortality in patients with acute myocardial infarction. 2. **Thrombolytic treatment in acute massive pulmonary embolism with haemodynamic instability.** The diagnosis should be confirmed whenever possible by objective means such as pulmonary angiography or non-invasive procedures such as lung scanning. There are no clinical trials on mortality and late morbidity related to pulmonary embolism. 3. **Thrombolytic treatment of acute ischaemic stroke** Treatment must be started as early as possible within 4.5 hours after onset of stroke symptoms and after exclusion of intracranial haemorrhage by appropriate imaging techniques (e.g. cranial computerised tomography or other diagnostic imaging method sensitive for the presence of haemorrhage). The treatment effect is time-dependent; therefore earlier treatment increases the probability of a favourable outcome.
**4.3 Contraindications** ACTILYSE is contraindicated in - patients with known hypersensitivity to the active substance alteplase or to any of the excipients - cases where there is a high risk of haemorrhage such as: - significant bleeding disorder at present or within the past 6 months, known haemorrhagic diathesis - patients receiving effective oral anticoagulants treatment, e.g. warfarin sodium (INR> 1.3) (please see section Special warnings and precautions, subsection “Bleeding” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - any history of central nervous system damage (i.e. neoplasm, aneurysm, intracranial or spinal surgery) - history or evidence or suspicion of intracranial haemorrhage including sub-arachnoid haemorrhage - severe uncontrolled arterial hypertension - major surgery or significant trauma in the past 10 days (this includes any trauma associated with the current acute myocardial infarction), recent trauma to head or cranium - prolonged or traumatic cardiopulmonary resuscitation (> 2 minutes), obstetrical delivery, within the past 10 days, recent puncture of a non-compressible blood-vessel (e.g. subclavian or jugular vein puncture) - severe hepatic dysfunction, including hepatic failure, cirrhosis, portal hypertension (oesophageal varices) and active hepatitis - bacterial endocarditis, pericarditis - acute pancreatitis - documented ulcerative gastro-intestinal disease during the last 3 months - arterial aneurysms, arterial/venous malformations - neoplasm with increased bleeding risk In the indication of acute myocardial the following contraindications apply in addition: - Haemorrhagic stroke or stroke of unknown origin at any time - Ischaemic stroke or transient ischaemic attack (TIA) in the preceding 6 months, except current acute ischaemic stroke within 4.5 hours In the indication of acute massive pulmonary embolism the following contraindications apply in addition: - Haemorrhagic stroke or stroke of unknown origin at any time - Ischaemic stroke or transient ischaemic attack (TIA) in the preceding 6 months, except current acute ischaemic stroke within 4.5 hours In the indication acute ischaemic stroke the following contraindications apply in addition: - symptoms of ischaemic attack began more than 4.5 hours prior to infusion start or when time of symptom onset is unknown - symptoms of acute ischaemic stroke that were either rapidly improving or only minor before start of infusion - severe stroke as assessed clinically (e.g. NIHSS > 25) and/or by appropriate imaging techniques - seizure at the onset of stroke - history of previous stroke or serious head-trauma within three months - a combination of previous stroke and diabetes mellitus - administration of heparin within 48 hours preceding the onset of stroke with an elevated activated partial thromboplastin time (aPTT) at presentation - platelet count of less than 100,000 / mm3 - systolic blood pressure > 185 mmHg or diastolic blood pressure > 110 mmHg, or aggressive management (IV medication) necessary to reduce blood pressure to these limits - blood glucose < 50 mg/dL or > 400 mg/dL (< 2.8 mmol/L or > 22.2 mmol/L) - children under 16 years of age (for children ≥ 16 years of age see section Special warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_)
B01AD02
alteplase
Manufacturer Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
BOEHRINGER INGELHEIM PHARMA GMBH & CO KG
Active Ingredients
RECOMBINANT HUMAN TISSUE-TYPE PLASMINOGEN ACTIVATOR
50 mg/vial
Documents
Package Inserts
Actilyse_PI.pdf
Approved: July 19, 2022
