Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SOLUTION
**4.2 Posology and method of administration** **SPECIAL POPULATIONS** Patients with hepatic or renal impairment COMBIVENT® has not been studied in patients with hepatic or renal insufficiency. It should be used with caution in those patient populations. Paediatric population Because of insufficient information in children COMBIVENT® is not indicated for paediatric patients. Patients should be advised to consult a physician or the nearest hospital immediately in the case of acute or rapidly worsening dyspnoea if additional inhalations of COMBIVENT® do not produce an adequate improvement. If higher than recommended doses of COMBIVENT® are required to control symptoms, the patient’s therapy plan should be reviewed. In asthma, concomitant anti-inflammatory therapy should be considered. The following doses of COMBIVENT® are recommended for adults (including elderly patients): COMBIVENT® nebuliser solution in unit dose vials (UDVs) COMBIVENT® nebuliser solution in unit dose vials may be administered from a suitable nebuliser or an intermittent positive pressure ventilator. Treatment should be initiated and administered under medical supervision, e.g. in the hospital setting. Home based treatment can be recommended in exceptional cases (severe symptoms or experienced patients requiring higher doses) when a low dose rapid acting beta-agonist bronchodilator has been insufficient in providing relief after consultation with an experienced physician. The treatment with the nebuliser solution in UDVs should always be started with the lowest recommended dose (1 UDV). In very severe cases two unit dose vials may be required for symptom relief. Administration should be stopped when sufficient symptom relief is achieved. **Treatment of acute attacks:** 1 unit dose vial is sufficient for prompt symptom relief in many cases. In severe cases if an attack has not been relieved by one unit dose vial, the administration of a second unit dose vial may be required. Patients should be advised to consult the physician or the nearest hospital immediately in these cases. **Maintenance treatment:** 1 unit dose vial three or four times daily. Instructions for use The unit dose vials are intended only for inhalation with suitable nebulising devices and must not be taken orally or administered parenterally. The content of the unit dose vials does not need to be diluted for nebulization. 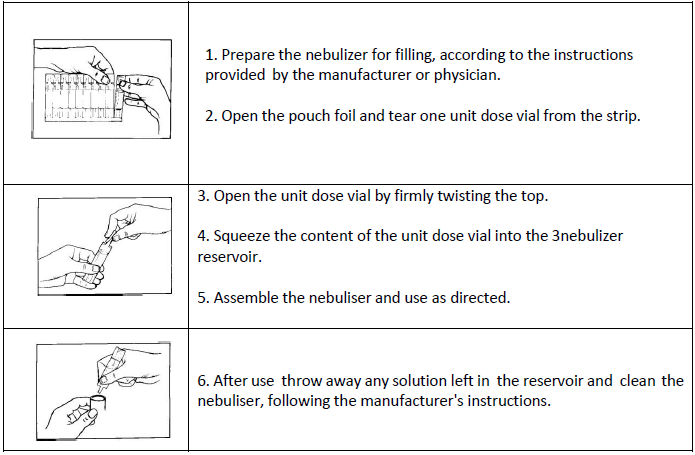 Since the unit dose vials contain no preservative, it is important that the contents are used soon after opening and that a fresh vial is used for each administration to avoid microbial contamination. Partly used, opened or damaged unit dose vials should be discarded. It is strongly recommended not to mix COMBIVENT® nebuliser solution with other drugs in the same nebuliser.
NASAL
Medical Information
**4.1 Therapeutic indications** COMBIVENT® nebuliser solution in unit dose vials are indicated for the management of reversible bronchospasm associated with obstructive airway diseases in patients who require more than a single bronchodilator.
**4.3 Contraindications** COMBIVENT® is contraindicated in patients with hypertrophic obstructive cardiomyopathy or tachyarrhythmia. COMBIVENT® is also contraindicated in patients with a history of hypersensitivity to atropine or its derivatives or to any other component of the product.
R03AK04
salbutamol and sodium cromoglicate
Manufacturer Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
LABORATOIRE UNITHER
Active Ingredients
IPRATROPIUM BROMIDE MONOHYDRATE 0.52MG/2.5ML EQV. TO IPRATROPIUM BROMIDE ANHYDROUS
0.5 mg/2.5 ml
Documents
Package Inserts
Combivent unit dose vial for inhalation PI.pdf
Approved: November 14, 2019
