Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**Dosage and Administration** **Dosage – Adults** _**Plaque Psoriasis**_ For the treatment of plaque psoriasis, STELARA® is administered by subcutaneous injection. STELARA® is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of psoriasis. 1. For patients weighing ≤ 100 kg, the recommended dose is 45 mg initially and 4 weeks later, followed by dosing every 12 weeks. 2. For patients weighing > 100 kg, alternatively a dose of 90 mg initially and 4 weeks later, followed by dosing every 12 weeks may be used. In patients weighing > 100 kg, 45mg was also shown to be efficacious. However, 90mg resulted in greater efficacy in these patients. _**Re-treatment**_ Re-treatment with a dosing regimen of Weeks 0 and 4, followed by 12 weeks dosing after interruption of therapy has been shown to be safe and effective. _**Psoriatic Arthritis**_ For the treatment of psoriatic arthritis, STELARA® is administered by subcutaneous injection. The recommended dose of STELARA® is 45 mg administered at Weeks 0 and 4, then every 12 weeks thereafter. Alternatively, 90 mg may be used in patients with a body weight greater than 100 kg. Consideration should be given to discontinuing treatment in patients who have shown no response up to 28 weeks of treatment. _**Crohn’s Disease and Ulcerative Colitis**_ In patients with Crohn’s disease and ulcerative colitis, the recommended treatment regimen is a single intravenous (IV) tiered dose of STELARA® based on body weight (Table 1), followed by 90 mg subcutaneous dosing 8 weeks later, then every 8 weeks thereafter (see _Instructions for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 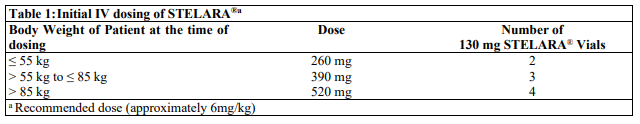 For some patients, a single IV dose based on body weight (Table 1) followed by 90 mg subcutaneous dosing 8 weeks later, then every 12 weeks thereafter may be acceptable according to clinical judgment. Patients who inadequately respond to 90 mg subcutaneous dosing every 12 weeks may benefit from an increase in dosing frequency to every 8 weeks (see _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Immunomodulators and/or corticosteroids may be continued during treatment with STELARA®. In patients who have responded to treatment with STELARA® corticosteroids may be reduced or discontinued in accordance with standard of care. If therapy in Crohn’s disease or Ulcerative Colitis is interrupted, resumption of treatment with subcutaneous dosing every 8 weeks is safe and effective. **Dosage – Pediatric Population (6 years and older)** _**Plaque Psoriasis**_ For the treatment of plaque psoriasis, STELARA® should be administered by subcutaneous injection. The recommended dose of STELARA® based on body weight is shown below (Table 2). STELARA® should be administered at week 0 and 4, then every 12 weeks thereafter. 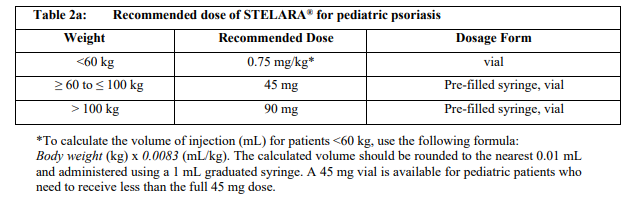 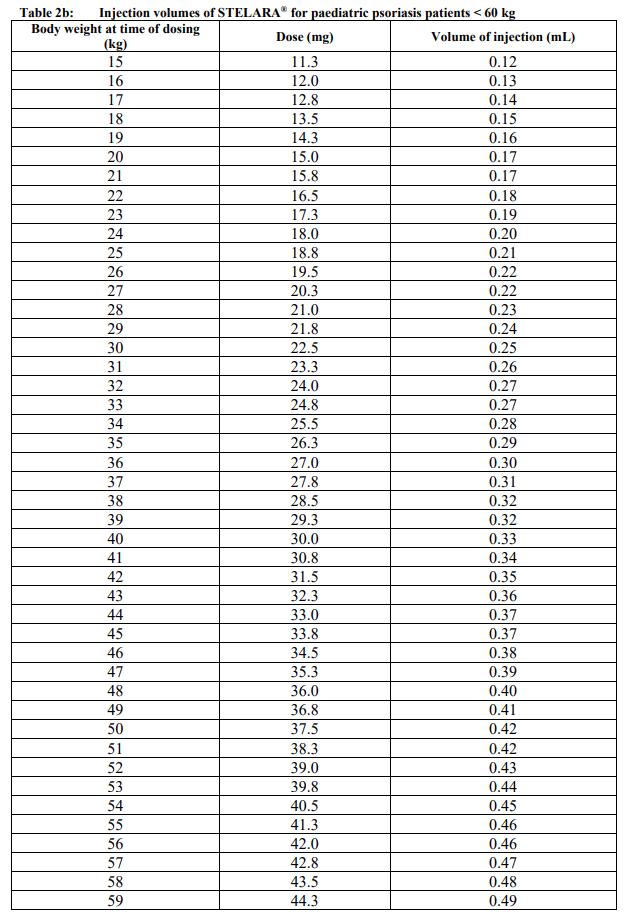 **General Consideration for Administration** _**Subcutaneous administration**_ STELARA® is intended for use under the guidance and supervision of a physician. In pediatric patients, it is recommended that STELARA® be administered by a health-care provider. Patients or their caregivers may inject STELARA® if a physician determines that it is appropriate and with medical follow-up as necessary, after proper training in subcutaneous injection technique and disposal (see _Instructions for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Comprehensive instructions for the subcutaneous administration of STELARA® are given in the “Patient Information Leaflet”. Patients should be instructed to inject the prescribed amount of STELARA® according to the directions provided in the patient information leaflet. The needle cover on the pre-filled syringe contains dry natural rubber (a derivative of latex), which may cause allergic reactions in individuals sensitive to latex. Prior to subcutaneous administration, visually inspect the solution in the STELARA® vial for particulate matter and discoloration. The product is colorless to light yellow and may contain a few small translucent or white particles of protein. This appearance is not unusual for proteinaceous solutions. The product should not be used if solution is discolored or cloudy, or if other particulate matter is present. STELARA® does not contain preservatives; therefore any unused product remaining in the vial and syringe should not be used. _**Intravenous infusion (Crohn’s Disease and Ulcerative Colitis)**_ STELARA® 130 mg vial is for IV infusion only. Intravenous infusion of STELARA® should be administered by qualified health-care professionals (for preparation, see _Instructions for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Special populations** _**Pediatrics**_ Studies of STELARA® in pediatric patients below 6 years of age have not been conducted. No studies have been conducted in pediatric patients with psoriatic arthritis, Crohn’s disease or ulcerative colitis. _**Elderly**_ Of the 6709 patients exposed to STELARA®, a total of 353 were 65 years or older (183 patients with psoriasis, 69 patients with psoriatic arthritis, 58 with Crohn’s disease, and 43 patients with ulcerative colitis). No major age-related differences in clearance or volume of distribution were observed in clinical studies. Although no overall differences in safety or efficacy were observed between older and younger patients in clinical studies in approved indications, the number of patients aged 65 and over is not sufficient to determine whether they respond differently from younger patients. _**Renal impairment**_ Specific studies have not been conducted in patients with renal insufficiency. _**Hepatic impairment**_ Specific studies have not been conducted in patients with hepatic insufficiency.
INTRAVENOUS
Medical Information
**Indications** **Plaque Psoriasis:** STELARA® is indicated for the treatment of adult patients (18 years or older) with moderate to severe plaque psoriasis who failed to respond to, or who have a contraindication to, or are intolerant to other systemic therapies including cyclosporin, methotrexate or PUVA. **Pediatric Plaque Psoriasis:** STELARA® is indicated for the treatment of pediatric patients (children and adolescents) (6 years and older) with moderate to severe plaque psoriasis who are inadequately controlled by, or are intolerant to, other systemic therapies or phototherapies. **Psoriatic Arthritis (PsA):** STELARA®, alone or in combination with methotrexate (MTX), is indicated for: - the treatment of adult patients (18 years or older) with active psoriatic arthritis when the response to previous non-biological disease-modifying anti-rheumatic drug (DMARD) therapy has been inadequate - inhibiting the progression of structural damage **Crohn’s Disease:** STELARA® is indicated for the treatment of adults with moderately to severe active Crohn’s disease who have: - failed or were intolerant to treatment with immunomodulators or corticosteroids, but never failed treatment with a tumor necrosis factor (TNF) blocker or - failed or were intolerant to treatment with one or more TNF blockers **Ulcerative Colitis:** STELARA® is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic or have medical contraindications to such therapies.
**Contraindications** Severe hypersensitivity to ustekinumab or to any of the excipients (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L04AC05
ustekinumab
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Cilag AG
