Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Therapy should be initiated by a physician experienced in the diagnosis and treatment of patients with leukaemia. Haematologic support such as platelet transfusion and haematopoietic growth factors can be used during treatment if clinically indicated. Before starting treatment with ponatinib, the cardiovascular status of the patient should be assessed, including history and physical examination, and cardiovascular risk factors should be actively managed. Cardiovascular status should continue to be monitored and medical and supportive therapy for conditions that contribute to cardiovascular risk should be optimised during treatment with ponatinib. Posology The recommended starting dose is 45 mg of ponatinib once daily. For the standard dose of 45 mg once daily, a 45 mg film-coated tablet is available. Treatment should be continued as long as the patient does not show evidence of disease progression or unacceptable toxicity. Patients should be monitored for response according to standard clinical guidelines. Discontinuing ponatinib should be considered if a complete haematologic response has not occurred by 3 months (90 days). The risk of arterial occlusive events is likely to be dose-related. Reducing the dose of Iclusig to 15 mg should be considered for CP-CML patients who have achieved a major cytogenetic response taking the following factors into account in the individual patient assessment: cardiovascular risk, side effects of ponatinib therapy, time to response, and BCR-ABL transcript levels (see sections 4.4 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If dose reduction is undertaken, close monitoring of response is recommended. In patients with loss of response the dose of Iclusig can be re-escalated to a previously tolerated dosage of 30 mg or 45 mg orally once daily. Management of toxicities Dose modifications or interruption of dosing should be considered for the management of haematological and non-haematological toxicities. In the case of severe adverse reactions, treatment should be withheld. For patients whose adverse reactions are resolved or attenuated in severity, Iclusig may be restarted and escalation of the dose back to the daily dose used prior to the adverse reaction may be considered, if clinically appropriate. For a dose of 30 mg or 15 mg once daily, 15 mg film-coated tablets are available. _Myelosuppression_ Dose modifications for neutropenia (ANC\* < 1.0 x 109/L) and thrombocytopenia (platelet < 50 x 109/L) that are unrelated to leukaemia are summarized in Table 1. 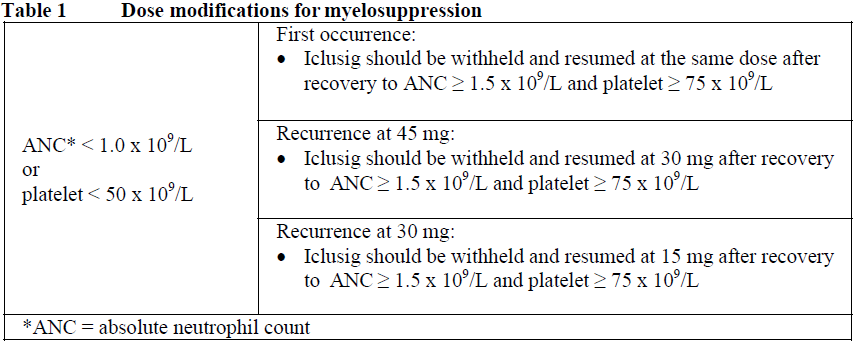 _Arterial occlusion and venous thromboembolism_ In a patient suspected of developing an arterial occlusive event or a venous thromboembolism, Iclusig should be immediately interrupted. A benefit-risk consideration should guide a decision to restart Iclusig therapy (see sections 4.4 and 4.8 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) after the event is resolved. Hypertension may contribute to risk of arterial occlusive events. Iclusig treatment should be temporarily interrupted if hypertension is not medically controlled. _Pancreatitis_ Recommended modifications for pancreatic adverse reactions are summarized in Table 2. 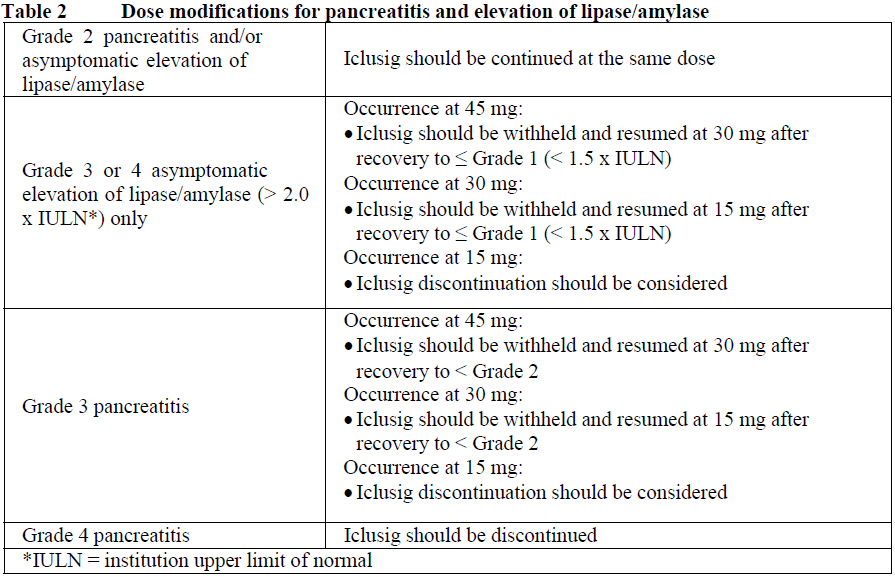 _Hepatic Toxicity_ Dose interruption or discontinuation may be required as described in Table 3. 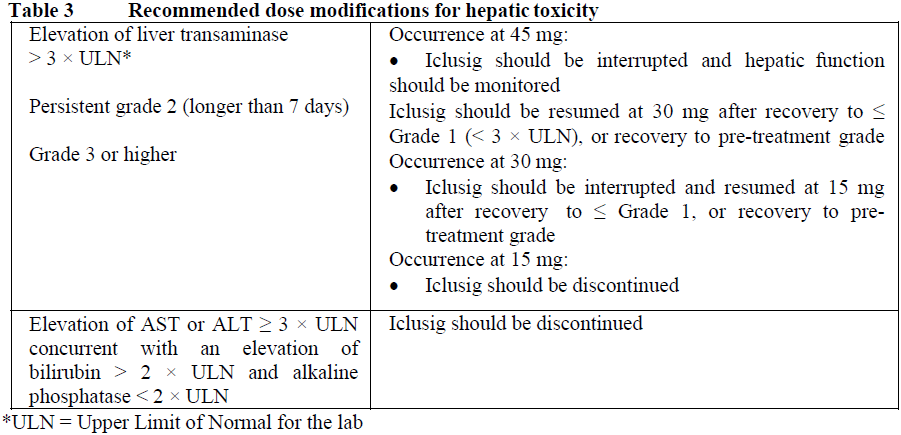 _Elderly patients_ Of the 449 patients in the clinical study of Iclusig, 155 (35%) were ≥ 65 years of age. Compared to patients < 65 years, older patients are more likely to experience adverse reactions. _Hepatic impairment_ Patients with hepatic impairment may receive the recommended starting dose. Caution is recommended when administering Iclusig to patients with hepatic impairment (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ Renal excretion is not a major route of ponatinib elimination. Iclusig has not been studied in patients with renal impairment. Patients with estimated creatinine clearance of ≥ 50 mL/min should be able to safely receive Iclusig with no dosage adjustment. Caution is recommended when administering Iclusig to patients with estimated creatinine clearance of < 50 mL/min, or end-stage renal disease. _Paediatric population_ The safety and efficacy of Iclusig in patients less than 18 years of age have not been established. No data are available. Method of administration Iclusig is for oral use. The tablets should be swallowed whole. Patients should not crush or dissolve the tablets. Iclusig may be taken with or without food. Patients should be advised not to swallow the desiccant canister found in the bottle.
ORAL
Medical Information
**4.1 Therapeutic indications** Iclusig is indicated in adult patients with - chronic phase, accelerated phase, or blast phase chronic myeloid leukaemia (CML) who are resistant to dasatinib or nilotinib; who are intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not clinically appropriate; or who have the T315I mutation. - Philadelphia chromosome positive acute lymphoblastic leukaemia (Ph+ ALL) who are resistant to dasatinib; who are intolerant to dasatinib and for whom subsequent treatment with imatinib is not clinically appropriate; or who have the T315I mutation. See sections 4.2 for the assessment of cardiovascular status prior to start of therapy and 4.4 for situations where an alternative treatment may be considered – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01EA05
ponatinib
Manufacturer Information
STEWARD CROSS PTE LTD
PATHEON INC.
