Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER, FOR SOLUTION
**2.2 DOSAGE AND ADMINISTRATION** Evrysdi oral solution must be constituted by a health care provider (HCP) prior to being dispensed. **General** SMA treatment should be initiated as early as possible after diagnosis. Evrysdi is taken orally once daily using the oral syringe provided, at approximately the same time each day. The recommended once daily dose of Evrysdi for SMA patients is determined by age and body weight (see Table 1). 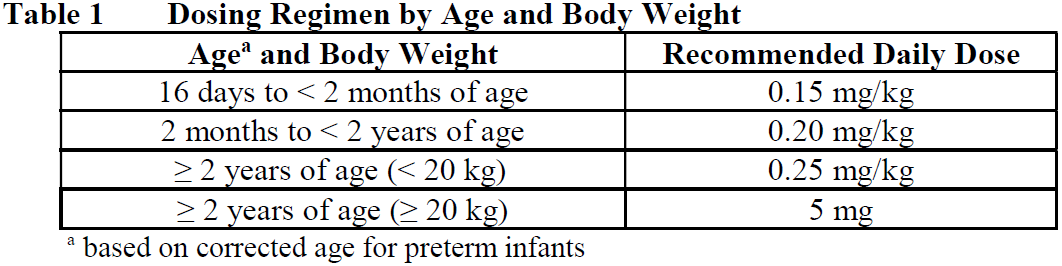 Dose changes must be made under the supervision of a HCP. Treatment with a daily dose above 5 mg has not been studied. No data are available in infants below 16 days of age. **Method of administration** Use the re-usable oral syringe provided to deliver the daily dose of Evrysdi. It is recommended a HCP discuss with the patient or caregiver how to prepare the prescribed daily dose prior to administration of the first dose (see section _4.2 Special Instruction for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The patient should drink water after taking Evrysdi to ensure the drug has been completely swallowed. If the patient is unable to swallow and has a nasogastric or gastrostomy tube, administer Evrysdi via the tube. The tube should be flushed with water after delivering Evrysdi (see section _4.2 Special Instructions for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Delayed or Missed Doses** Evrysdi is taken orally once daily at approximately the same time each day. If a dose of Evrysdi is missed, administer as soon as possible if still within 6 hours of the scheduled dose. Otherwise, skip the missed dose and take the next dose at the regularly scheduled time the next day. If a dose is not fully swallowed or vomiting occurs after taking a dose of Evrysdi, do not administer another dose to make up for the incomplete dose. Wait until the next day to administer the next dose at the regularly scheduled time. **2.2.1 Special Dosage Instructions** **Pediatric use** The safety and efficacy of Evrysdi in pediatric patients < 16 days of age have not yet been established (see section _3.1.2 Clinical / Efficacy Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The safety and efficacy of Evrysdi in preterm infants before reaching the corrected age of 16 days have not been established. **Geriatric use** The pharmacokinetics (PK) and safety of Evrysdi have been assessed in subjects without SMA up to 69 years of age. Evrysdi has not been studied in patients with SMA above 60 years of age (see sections _3.2.5 Pharmacokinetics in Special Populations_ and _2.5.5 Geriatric Use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal Impairment** The safety and efficacy of Evrysdi in patients with renal impairment have not been studied. No dose adjustment is expected to be required in patients with renal impairment (see sections _3.2.5 Pharmacokinetics in Special Populations_ and _2.5.6 Renal Impairment_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic Impairment** No dose adjustment is required in patients with mild or moderate hepatic impairment. Evrysdi has not been studied in patients with severe hepatic impairment (see sections _3.2.5 Pharmacokinetics in Special Populations_ and _2.5.7 Hepatic Impairment_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL, ENTERAL
Medical Information
**2.1 THERAPEUTIC INDICATION(S)** Evrysdi is indicated for the treatment of spinal muscular atrophy (SMA).
**2.3 CONTRAINDICATIONS** Evrysdi is contraindicated in patients with a known hypersensitivity to risdiplam or any of the excipients.
M09AX10
risdiplam
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
F. Hoffmann-La Roche Ltd
Active Ingredients
Documents
Package Inserts
Evrysdi PI.pdf
Approved: June 19, 2023
