Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**DOSAGE AND DIRECTIONS FOR USE** Children aged from 2 to 6 years: 2.5 mg twice daily \[5 drops twice daily or 2.5 ml of oral solution twice daily (half of a spoon twice daily)\]. Children aged from 6 to 12 years: 5 mg \[10 drops, or 5 ml oral solution (1 full spoon) or half of the tablet\] twice daily. Adolescents 12 years and above, and adults: 10 mg once daily \[1 tablet, 20 drops, or 10 ml oral solution (2 full spoons)\]. A 5 mg starting dose (half of the tablet, 10 drops, or 5 ml oral solution) may be proposed if this leads to satisfactory control of the symptoms. The tablets need to be swallowed with a glass of liquid. The drops have to be diluted in liquid, while the solution can be swallowed as such. Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function is normal. Patients with moderate to severe renal impairment: since cetirizine is mainly excreted via renal route, in cases no alternative treatment can be used, the dosing intervals must be individualised according to renal function. Refer to the following table and adjust the dose as indicated. Dosing Adjustments for Adult Patients with Impaired Renal Function 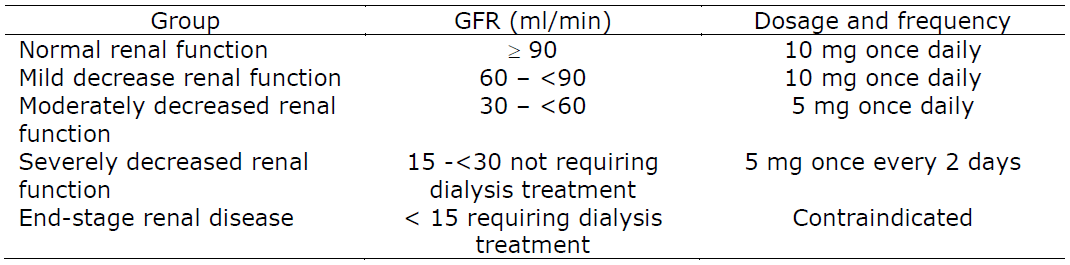 In paediatric patients suffering from renal impairment, the dose will have to be adjusted on an individual basis taking into account the renal clearance, age and body weight of the patient. Patients with hepatic impairment: no dose adjustment is needed in patients with solely hepatic impairment. Patients with hepatic impairment and renal impairment: adjustment of the dose is recommended (see _Patients with renal impairment above_).
ORAL
Medical Information
**INDICATIONS** For relief of: - nasal and ocular symptoms of seasonal and perennial allergic rhinitis; - symptoms of chronic idiopathic urticaria
**CONTRAINDICATIONS** Hypersensitivity to any of the constituents of the formulation, to hydroxyzine or to any piperazine derivatives. Patients with end-stage renal disease with GFR (Glomerular Filtration Rate) below 15 ml/min. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take cetirizine film-coated tablet. Patients with rare hereditary problems of fructose intolerance should not take cetirizine 1 mg/ml oral solution.
R06AE07
cetirizine
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
UCB FARCHIM S A
AESICA PHARMACEUTICALS S.R.L (PRIMARY AND SECONDARY PACKAGING)
Active Ingredients
Documents
Package Inserts
Zyrtec PI.pdf
Approved: March 28, 2023
