Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**4.2 Posology and method of administration** Treatment must be initiated and supervised by physicians experienced in the treatment of cancer. Posology - The recommended dose of Opdualag is 480 mg nivolumab and 160 mg relatlimab every 4 weeks administered as an intravenous infusion over 30–60 minutes. Treatment with Opdualag should be continued as long as clinical benefit is observed or until treatment is no longer tolerated by the patient. Dose escalation or reduction is not recommended. Dosing delay or discontinuation may be required based on individual safety and tolerability. Guidelines for permanent discontinuation or withholding of doses are described in Table 1. Detailed guidelines for the management of immune-related adverse reactions are described in section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. 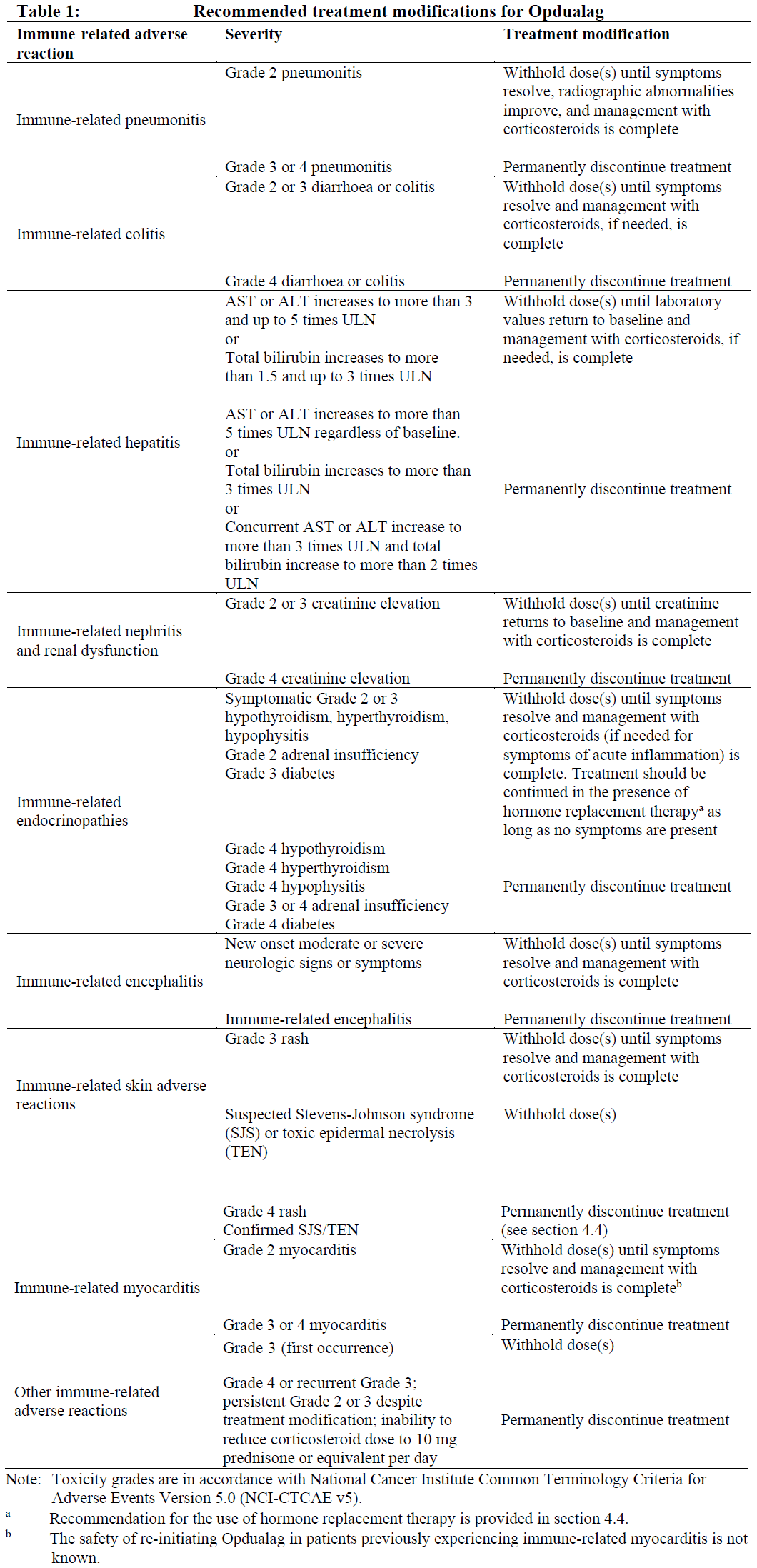 Opdualag should be permanently discontinued for: - Grade 4 or recurrent Grade 3 adverse reactions; - Persistent Grade 2 or 3 adverse reactions despite management; - Exceptions include endocrine adverse reactions and rash (see table 1 and section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Special populations_ _Paediatric population_ The safety and efficacy of Opdualag in children below 18 years of age have not been established. No data are available. _Elderly_ No dose adjustment is required for elderly patients (≥ 65 years) (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ No dose adjustment is required in patients with mild or moderate renal impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Data from patients with severe renal impairment are too limited to draw conclusions on this population. _Hepatic impairment_ No dose adjustment is required in patients with mild or moderate hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Data from patients with severe hepatic impairment are too limited to draw conclusions on this population. Method of administration Opdualag is for intravenous use only. It is to be administered as an intravenous infusion over a period of 30–60 minutes. Opdualag must not be administered as an intravenous push or bolus injection. Opdualag can be used without dilution, or may be diluted with sodium chloride 9 mg/mL (0.9%) solution for injection or glucose 50 mg/mL (5%) solution for injection (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For instructions on the preparation and handling of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Opdualag is indicated for the first-line treatment of unresectable or metastatic melanoma in adults with tumour cell PD-L1 expression < 1%.
**4.3 Contraindications** Hypersensitivity to the active substances or any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01XY03
xl 01 xy 03
Manufacturer Information
BRISTOL-MYERS SQUIBB (SINGAPORE) PTE. LTD.
Catalent Indiana LLC
Active Ingredients
Documents
Package Inserts
Opdualag Concentrate for Solution for Infusion PI.pdf
Approved: August 31, 2023
