Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Dosage and method of administration** **Recommended dose** The recommended daily dose of sorafenib is 400 mg (2 x 200 mg tablets) taken twice a day, either without food or together with a moderate fat meal. **Method of administration** For oral use. To be swallowed with a glass of water. **Duration of treatment** Treatment should be continued until the patient is no longer clinically benefiting from therapy or until unacceptable toxicity occurs. **Dose titration, dose adjustment, special monitoring advice** **Dose Reduction for Hepatocellular Carcinoma and advanced Renal cell Carcinoma** Management of suspected adverse drug reactions may require temporary interruption and/or dose reduction of sorafenib therapy. When dose reduction is necessary during the treatment of hepatocellular carcinoma (HCC) and advanced renal cell carcinoma (RCC), the sorafenib dose should be reduced to two tablets of 200 mg once daily (see “Special Warnings and Precautions for Use” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dose Reduction for Differentiated Thyroid Carcinoma Management of suspected adverse drug reactions may require temporary interruption and/or dose reduction of sorafenib therapy. When dose reduction is necessary during the treatment of differentiated thyroid carcinoma, the sorafenib dose should be reduced to 600mg daily in divided doses (two tablets of 200mg and one tablet of 200mg twelve hours apart). If additional dose reduction is necessary, sorafenib may be reduced to one tablet of 200mg twice daily, followed by one tablet of 200mg once daily. After improvement of non-hematological adverse reactions, the dose of sorafenib may be increased. 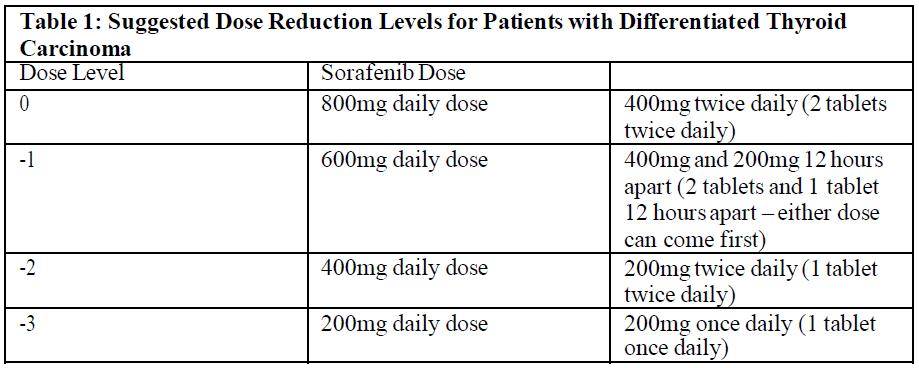 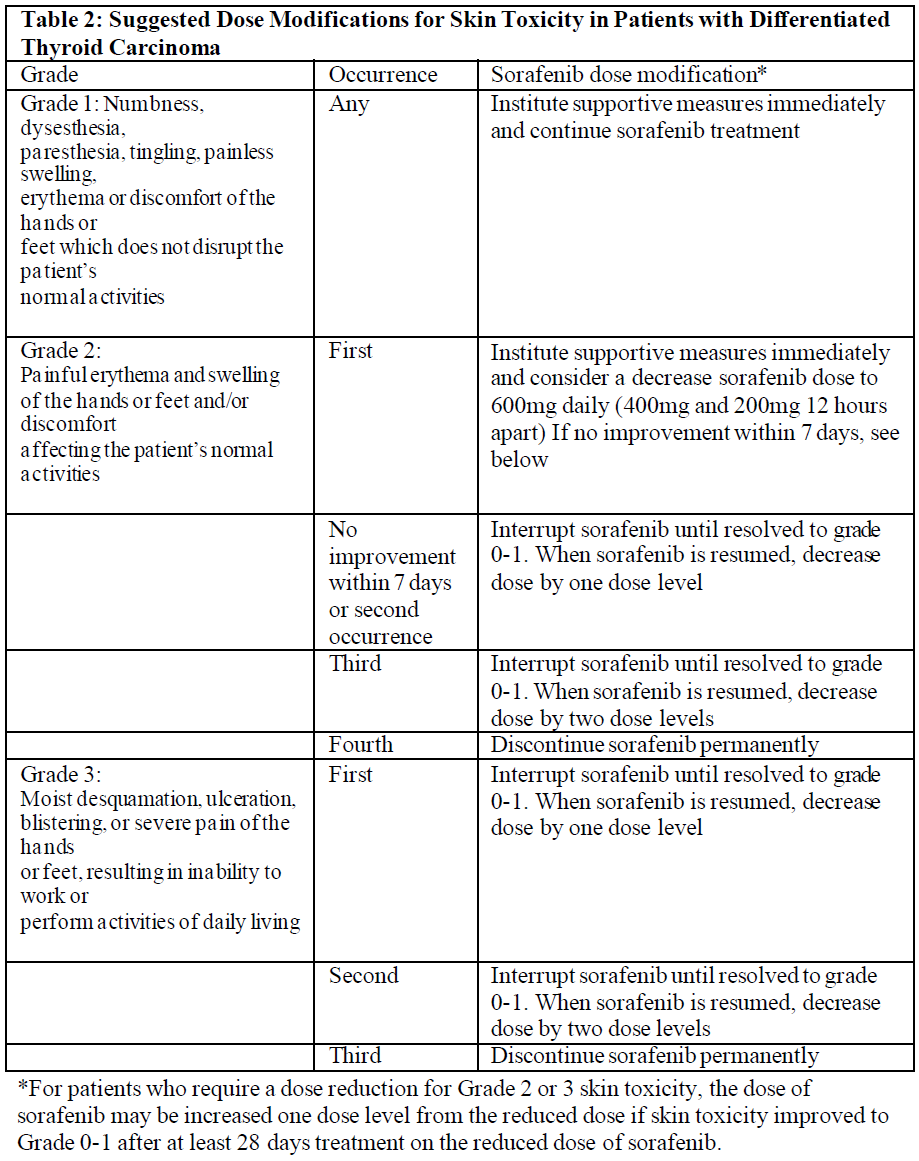 **Special Populations** **Pediatric Patients** The safety and effectiveness of sorafenib in paediatric patients has not been established. **Elderly (above 65 years), Gender and Body Weight** No dose adjustment is required on the basis of patient age (above 65 years), gender, or body weight. **Hepatic impairment** No dose adjustment is required in patients with Child-Pugh A or B hepatic impairment. Sorafenib has not been studied in patients with Child-Pugh C hepatic impairment (see “Pharmacokinetics Properties” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Renal impairment** No dose adjustment is required in patients with mild, moderate, or severe renal impairment not requiring dialysis. Sorafenib has not been studied in patients undergoing dialysis (see “Pharmacokinetics Properties” – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Monitoring of fluid balance and electrolytes in patients at risk of renal dysfunction is advised.
ORAL
Medical Information
**4.1 Indication(s)** NEXAVAR is indicated for the treatment of patients with unresectable hepatocellular carcinoma (HCC). NEXAVAR is indicated for the treatment of patients with advanced renal cell carcinoma who have failed prior systemic therapy or are considered unsuitable for such therapy. NEXAVAR is indicated for the treatment of patients with locally advanced or metastatic, progressive, differentiated thyroid carcinoma refractory to radioactive iodine treatment.
**4.3 Contraindications** Sorafenib is contraindicated in patients with known severe hypersensitivity to sorafenib or any of the excipients in the tablet.
L01XE05
xl 01 xe 05
Manufacturer Information
BAYER (SOUTH EAST ASIA) PTE LTD
BAYER AG
Active Ingredients
Documents
Package Inserts
Nexavar Tablet PI.pdf
Approved: December 23, 2022
