Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**Posology and method of administration** The dosage for adults is 24–48 mg divided over the day. 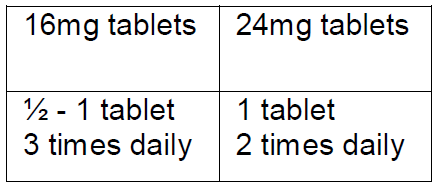 Should be swallowed with water. The dosage should be individually adapted according to the response. Improvement can sometimes only be observed after a couple of weeks of treatment. The best results are sometimes obtained only after a few months. There are indications that treatment from the onset of the disease prevents its progression and/or the loss of hearing in later phases of the disease. Pediatric population: Betaserc is not recommended for use in children under the age of 18 years due to insufficient data on safety and efficacy.
ORAL
Medical Information
**Therapeutic Indications** Ménière’s Syndrome as defined by the following core symptoms: - vertigo (with nausea/vomiting) - hearing loss (hardness of hearing) - tinnitus (ringing in the ears) Symptomatic treatment of vestibular vertigo.
**Contraindications** Hypersensitivity to the active substance or to any of the excipients. Phaeochromocytoma.
N07CA01
betahistine
Manufacturer Information
ABBOTT LABORATORIES (SINGAPORE ) PRIVATE LIMITED
Mylan Laboratories SAS
Active Ingredients
Documents
Package Inserts
Betaserc tablets PI.pdf
Approved: January 24, 2018
