Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, DELAYED RELEASE
**4.2. Posology and method of administration** **_Pantobex Delayed Release Tablet 20_ mg** Pantobex 20 mg delayed-release tablets should not be chewed or crushed, and should be swallowed whole with water. 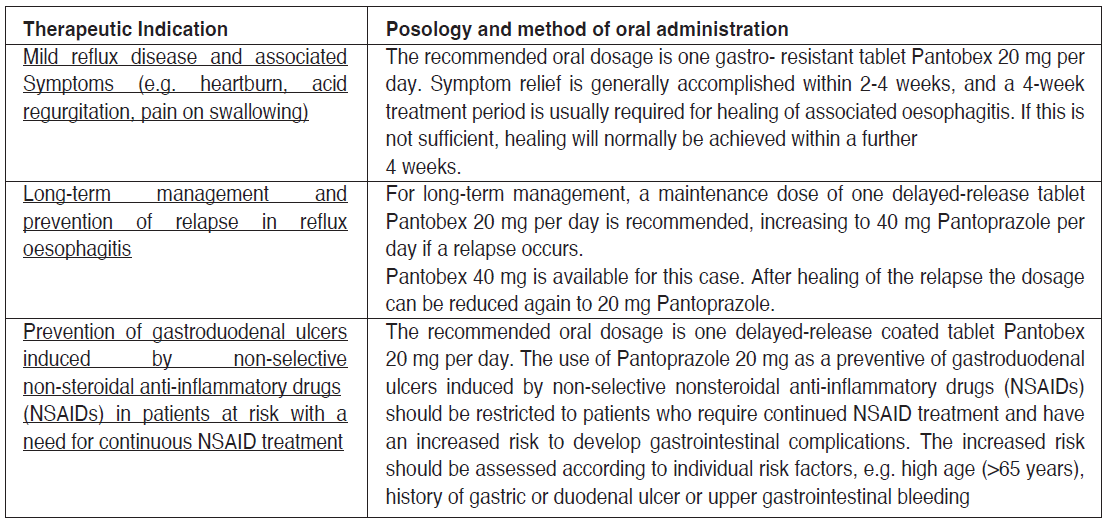 **_Pantobex Delayed Release Tablet 40_ mg** Pantobex 40 mg delayed-release tablets should not be chewed or crushed, and should be swallowed whole with water.  **Special Patient Populations** _**Pediatric patients:**_ The experience in children is limited. Pantoprazole 20 mg and 40 mg tablet is not recommended for use in children below 12 years of age due to limited data on safety and efficacy in this age group. Pantoprazole 40 mg powder for solution for injection is not recommended for use in patients below 18 years of age. _**Impaired hepatic function:**_ A daily dose of Pantoprazole 20 mg should not be exceeded in patients with severe liver impairment (See section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In addition, Pantoprazole 40 mg tablet must not be used in combination treatment (e.g. amoxicillin, clarithromycin,) for eradication of H. pylori in patients with moderate to severe hepatic dysfunction since currently no data are available on the efficacy and safety of pantoprazole in combination treatment of these patients (See section 4.3). _**Impaired renal function:**_ No dose adjustment is necessary in those with impaired renal function. In addition, pantoprazole 40 mg tablet must not be used in combination treatment (e.g. amoxicillin, clarithromycin) for eradication of H. pylori in patients with impaired renal function, since currently no data are available on the efficacy and safety of pantoprazole in combination treatment for these patients. _**Elderly patients:**_ Generally, no dose adjustment is necessary in elderly patients. However, the daily dose of 40mg should not be exceeded in treatment of gastric or duodenal ulcer.
ORAL
Medical Information
**4.1. Therapeutic indications** **_Pantobex Delayed Release Tablet 20_ mg** - Mild reflux disease and associated symptoms (e.g. heartburn, acid regurgitation, pain on swallowing). - Long-term management and prevention of relapse in reflux oesophagitis. - Prevention of gastroduodenal ulcers induced by non-selective non-steroidal anti- inflammatory drugs (NSAIDs) in patients at risk with a need for continuous NSAID treatment. **_Pantobex Delayed Release Tablet 40_ mg** - In combination with two appropriate antibiotics (see Dosage) for the eradication of H.pylori in patients with peptic ulcers with the objective of reducing the recurrence of duodenal and gastric ulcers caused by this microorganism. - Duodenal ulcer - Gastric ulcer - Moderate and severe reflux oesophagitis
**4.3 Contraindications** Pantobex should generally not be used in cases of known hypersensitivity to one of the other constituents of Pantobex 20 mg, Pantobex 40 mg tablet or of the combination partners. Pantobex 40 mg tablet must not be used in combination treatment for eradication of H.pylori in patients with moderate to severe liver or kidney function disturbances since currently no clinical data are available on the efficacy and safety of Pantoprazole 40 mg in combination treatment of these patients.
A02BC02
pantoprazole
Manufacturer Information
GOLDPLUS UNIVERSAL PTE LTD
Beximco Pharmaceuticals Limited
Active Ingredients
Documents
Package Inserts
Pantobex Delayed Release Tablet PI.pdf
Approved: September 1, 2022
