Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**2 DOSAGE AND ADMINISTRATION** **2.1 Dosing Guidelines** - PRECEDEX dosing should be individualized and titrated to desired clinical response. - PRECEDEX is not indicated for infusions lasting longer than 24 hours. - PRECEDEX should be administered using a controlled infusion device. **2.2 Dosage Information** 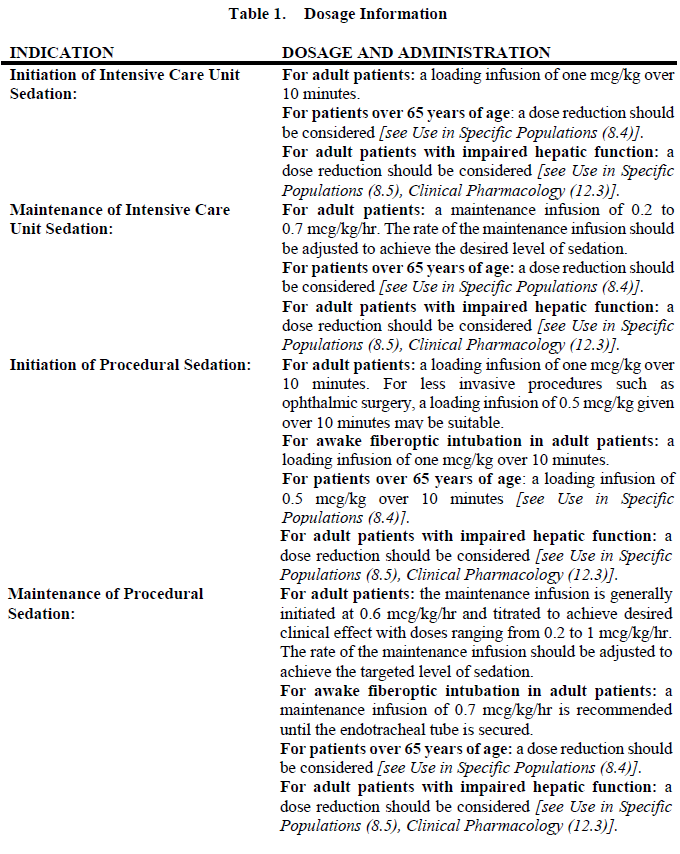 **2.3 Dosage Adjustment** Due to possible pharmacodynamic interactions, a reduction in dosage of PRECEDEX or other concomitant anesthetics, sedatives, hypnotics or opioids may be required when co-administered _\[see Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Dosage reductions may need to be considered for adult patients with hepatic impairment, and geriatric patients _\[see Warnings and Precautions (5.8), Use in Specific Populations (8.5), Clinical Pharmacology (12.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.4 Preparation of Solution** PRECEDEX must be diluted in 0.9% sodium chloride solution to achieve required concentration (4 mcg/mL) prior to administration. Preparation of solutions is the same, whether for the loading dose or maintenance infusion. Strict aseptic technique must always be maintained during handling of PRECEDEX. To prepare the infusion, withdraw 2 mL of PRECEDEX and add to 48 mL of 0.9% sodium chloride injection to a total of 50 mL. Shake gently to mix well. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if product is discolored or if precipitate matter is present. **2.5 Administration with Other Fluids** PRECEDEX infusion should not be co-administered through the same intravenous catheter with blood or plasma because physical compatibility has not been established. PRECEDEX has been shown to be incompatible when administered with the following drugs: amphotericin B, diazepam. PRECEDEX has been shown to be compatible when administered with the following intravenous fluids and drugs: 0.9% sodium chloride in water, 5% dextrose in water, 20% mannitol, alfentanil hydrochloride, amikacin sulfate, aminophylline, amiodarone hydrochloride, ampicillin sodium, ampicillin sodium-sulbactam sodium, atracurium besylate, atropine sulfate, azithromycin, aztreonam, bretylium tosylate, bumetanide, butorphanol tartrate, calcium gluconate, cefazolin sodium, cefepime hydrochloride, cefoperazone sodium, cefotaxime sodium, cefotetan sodium, cefoxitin sodium, ceftazidime, ceftizoxime sodium, ceftriaxone sodium, cefuroxime sodium, chlorpromazine hydrochloride, cimetidine hydrochloride, ciprofloxacin, cisatracurium besylate, clindamycin phosphate, dexamethasone sodium phosphate, digoxin, diltiazem hydrochloride, diphenhydramine hydrochloride, dobutamine hydrochloride, dolasetron mesylate, dopamine hydrochloride, doxycycline hyclate, droperidol, enalaprilat, ephedrine hydrochloride, epinephrine hydrochloride, erythromycin lactobionate, esmolol, etomidate, famotidine, fenoldopam mesylate, fentanyl citrate, fluconazole, furosemide, gatifloxacin, gentamicin sulfate, glycopyrrolate bromide, granisetron hydrochloride, haloperidol lactate, heparin sodium, hydrocortisone sodium succinate, hydromorphone hydrochloride, hydroxyzine hydrochloride, inamrinone lactate, isoproterenol hydrochloride, ketorolac tromethamine, labetalol, lactated Ringer’s solution, levofloxacin, lidocaine hydrochloride, linezolid, lorazepam, magnesium sulfate, meperidine hydrochloride, methylprednisolone sodium succinate, metoclopramide hydrochloride, metronidazole, midazolam, milrinone lactate, mivacurium chloride, morphine sulfate, nalbuphine hydrochloride, nitroglycerin, norepinephrine bitartrate, ofloxacin, ondansetron hydrochloride, pancuronium bromide, phenylephrine hydrochloride, piperacillin sodium, piperacillin sodium-tazobactam sodium, potassium chloride, procainamide hydrochloride, prochlorperazine edisylate, promethazine hydrochloride, propofol, ranitidine hydrochloride, rapacuronium bromide, remifentanil hydrochloride, rocuronium bromide, sodium bicarbonate, sodium nitroprusside, succinylcholine, sufentanil citrate, sulfamethoxazole-trimethoprim, theophylline, thiopental sodium, ticarcillin disodium, ticarcillin disodium-clavulanate potassium, tobramycin sulfate, vancomycin hydrochloride, vecuronium bromide, verapamil hydrochloride, and a plasma-substitute. **2.6 Compatibility with Natural Rubber** Compatibility studies have demonstrated the potential for absorption of PRECEDEX to some types of natural rubber. Although PRECEDEX is dosed to effect, it is advisable to use administration components made with synthetic or coated natural rubber gaskets.
INTRAVENOUS
Medical Information
**1 INDICATIONS AND USAGE** **1.1 Intensive Care Unit Sedation** PRECEDEX is indicated for sedation of initially intubated and mechanically ventilated patients during treatment in an intensive care setting. PRECEDEX should be administered by continuous infusion not to exceed 24 hours. PRECEDEX has been continuously infused in mechanically ventilated patients prior to extubation, during extubation, and post-extubation. It is not necessary to discontinue PRECEDEX prior to extubation. **1.2 Procedural Sedation** PRECEDEX is indicated for sedation of non-intubated patients prior to and/or during surgical and other procedures.
**4 CONTRAINDICATIONS** None
N05CM18
dexmedetomidine
Manufacturer Information
PFIZER PRIVATE LIMITED
HOSPIRA INC
Active Ingredients
Documents
Package Inserts
Precedex Infusion PI.pdf
Approved: February 13, 2023
