Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**2\. DOSAGE AND ADMINISTRATION** For Myocardial Imaging: The suggested dose range for I.V. administration of STAMICIS in a single dose to be employed in the average patient (70 Kg) is 370–1110 MBq (10–30 mCi). For Breast Imaging: The recommended dose range for I.V. administration of STAMICIS is a single dose of 740–1110 MBq (20 – 30 mCi). For Assessment of global ventricular function: 600–800 MBq injected as a bolus. For Localisation of hyperfunctioning parathyroid tissue: 200–700 MBq injected as a bolus. The typical activity is between 500–700 MBq. Renal impairment : Careful consideration of the activity to be administered is required since an increased radiation exposure is possible in these patients. Hepatic impairment :In general, activity selection for patients with a decreased hepatic function should be cautious, usually starting at the low end of the dosing range. **2.1 Image Acquisition** Breast Imaging: It is recommended that images are obtained with a table overlay to separate breast tissue from the myocardium and liver, and to exclude potential activity that may be present in the opposite breast. For lateral images, position the patient prone with the isolateral arm comfortably above the head, shoulders flat against the table, head turned to the side and relaxed, with the breast imaged pendent through an overlay cutout. The breast should not be compressed on the overlay. For anterior images, position the patient supine with both arms behind the head. For either lateral or anterior images, shield the chest and abdominal organs, or remove them from the field of view. For complete study, sets of images should be obtained five minutes after the injection, and in the following sequence: Beginning five minutes after the injection of Technetium Tc99m Sestamibi: - ten-minute lateral image of breast with abnormality - ten-minute lateral image of contralateral breast - ten-minute anterior image of both breasts Cardiac imaging Imaging should begin approximately after 30–60 min after injection to allow for hepatobiliary clearance. Longer delay can be required for resting images and for stress with vasodilatators alone because of the risk of higher subdiaphragmatic technetium (99mTc) activity. There is no evidence for significant changes in myocardial tracer concentration or redistribution, therefore imaging for up to 6 hours post injection is possible. Test may be done in a one day or two days protocol. Preferably tomographic imaging (SPECT) with or without ECG gating should be performed. Parathyroid imaging Parathyroid image acquisition depends on the protocol chosen. The most used studies are either the subtraction and/or the dual-phase techniques, which can be performed together. For the subtraction technique either sodium iodide (123I) or sodium pertechnetate (99mTc) can be used for imaging for the thyroid gland since these radiopharmaceuticals are trapped by functioning thyroid tissue. This image is subtracted from the technetium (99mTc) sestamibi image, and pathological hyperfunctioning parathyroid tissue remains visible after subtraction. When sodium iodide (123I) is used, 10 to 20 MBq are orally administered. Four hours after the administration, neck and thorax images may be obtained. After sodium iodide (123I) image acquisition, 200 to 700 MBq of technetium (99mTc) sestamibi are injected and images are acquired 10 minutes post injection in double acquisition”with 2 peaks of gamma energy (140 keV for technetium (99mTc) and 159 keV for iodine (123I)). When sodium pertechnetate (99mTc) is used, 40–150 MBq are injected and neck and thorax images are acquired 30 minutes later. Then 200 to 700 MBq of technetium (99mTc) sestamibi are injected and a second acquisition of images is acquired 10 minutes later. When the dual phase technique is used, 400 to 700 MBq of technetium (99mTc) sestamibi are injected and the first neck and mediastinum image is obtained 10 minutes later. After a wash-out period of 1 to 2 hours, neck and mediastinum imaging is again performed. The planar images may be complemented by early and delayed SPECT or SPECT/CT. **2.2 Radiation Dosimetry** The radiation doses to organs and tissues of an average patient (70 Kg) per 1110 MBq (30 mCi) of Technetium Tc99m Sestamibi injected intravenously are shown in Table 1.0. 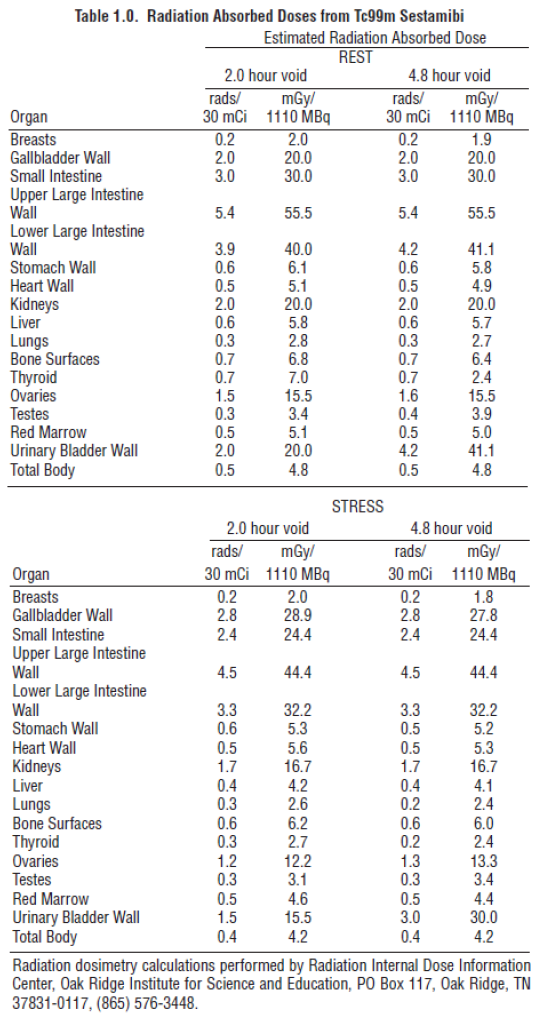 **2.3 Instructions For Preparation** Preparation of the Technetium Tc99m Sestamibi from the Kit for the Preparation of Technetium Tc99m Sestamibi is done by the following aseptic procedure: General Procedure: a. Prior to adding the Sodium Pertechnetate Tc99m Injection to the vial, inspect thevial carefully for the presence of damage, particularly cracks, and do not use the vial if found. Tear off a radiation symbol and attach it to the neck of the vial. b. Waterproof gloves should be worn during the preparation procedure. Remove the plastic disc from the vial and swab the top of the vial closure with alcohol to sanitize the surface. Boiling Water Bath Procedure: c. Place the vial in a suitable radiation shield with a fitted radiation cap. d. With a sterile shielded syringe, aseptically obtain additive-free, sterile, non-pyrogenic Sodium Pertechnetate Tc99m Injection \[925–5550 MBq, (25–150 mCi)\] in approximately 1 to 3 mL. e. Aseptically add the Sodium Pertechnetate Tc99m Injection to the vial in the lead shield. Without withdrawing the needle, remove an equal volume of headspace to maintain atmospheric pressure within the vial. f. Shake vigorously, about 5 to 10 quick upward-downward motions. g. Remove the vial from the lead shield and place upright in an appropriately shielded and contained boiling water bath, such that the vial is suspended above the bottom of the bath, and boil for 10 minutes. Timing for 10 minutes is begun as soon as the water begins to boil again. Do not allow the boiling water to come in contact with the aluminum crimp. h. Remove the vial from the water bath, place in the lead shield and allow to cool for fifteen minutes. Recon-o-Stat (thermal cycler) Procedure: c. Place the vial in the thermal cycler radiation shield. d. With a sterile shielded syringe, aseptically obtain additive-free, sterile, non-pyrogenic Sodium Pertechnetate Tc99m Injection \[925–5550 MBq, (25–150 mCi)\] in approximately 1 to 3 mL. e. Aseptically add the Sodium Pertechnetate Tc99m Injection to the vial in the lead shield. Without withdrawing the needle, remove an equal volume of headspace to maintain atmospheric pressure within the vial. f. Shake vigorously, about 5 to 10 quick upward-downward motions. g. Place shield on sample block. While slightly pressing downward, give the shield a quarter turn to make certain there is a firm fit between the shield and the sample block. h. Press the proceed button to initiate the program (the thermal cycler automatically heats & cools the vial and contents). Please see the Recon-o-Stat Instruction Manual for further details. General Procedure (cont.): i. Using proper shielding, the vial contents should be visually inspected. Use only if the solution is clear and free of particulate matter and discoloration. j. Assay the reaction vial using a suitable radioactivity calibration system. Record the Technetium Tc99m concentration, total volume, assay time and date, expiration time and lot number on the vial shield label and affix the label to the shield. k. Store the reaction vial containing the Technetium Tc99m Sestamibi at 15° to 25°C until use; at such time the product should be aseptically withdrawn. Technetium Tc99m Sestamibi should be used within six hours of preparation. The vial contains no preservative. Note: Adherence to the above product reconstitution instructions is recommended. The potential for cracking and significant contamination exists whenever vials containing radioactive material are heated. Product should be used within 6 hours after preparation. Final product with radiochemical purity of at least 90% was used in the clinical trials that established safety and effectiveness. The radiochemical purity was determined by the following method. **2.4 Determination of Radiochemical Purity in Technetium Tc99m Sestamibi** 1. Obtain a Baker-Flex Aluminum Oxide coated, plastic TLC plate, #1 B-F, pre-cut to 2.5 cm x 7.5 cm. 2. Dry the plate or plates at 100°C for 1 hour and store in a desiccator. Remove pre-dried plate from the desiccator just prior to use. 3. Apply 1 drop of ethanol\* using a 1 mL syringe with a 22–26 gauge needle, 1.5 cm from the bottom of the plate. THE SPOT SHOULD NOT BE ALLOWED TO DRY. 4. Add 2 drops of Technetium Tc99m Sestamibi solution, side by side on top of the ethanol\* spot. Return the plate to a desiccator and allow the sample spot to dry (typically 15 minutes). 5. The TLC tank is prepared by pouring ethanol\* to a depth of 3–4 mm. Cover the tank and let it equilibrate for ~10 minutes. 6. Develop the plate in the covered TLC tank in ethanol\* for a distance of 5 cm from the point of application. 7. Cut the TLC plate 4 cm from the bottom and measure the Tc99m activity in each piece by appropriate radiation detector. 8. Calculate the % Tc99m Sestamibi as:  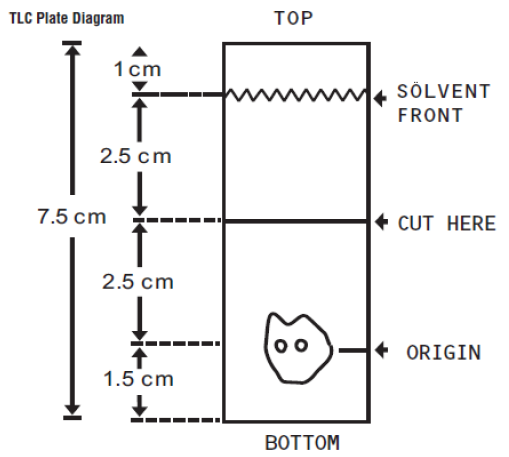 \*The ethanol used in this procedure should be 95% or greater. Absolute ethanol (99%) should remain at > 95% ethanol content for one week after opening if stored tightly capped, in a cool dry place.
INTRAVENOUS
Medical Information
**1\. INDICATIONS AND USAGE** _This medicinal product is for diagnostic use only. This is indicated for adults._ After radiolabelling with sodium pertechnetate (99mTc) solution, the solution of technetium (99mTc) sestamibi obtained is indicated for: - **Myocardial perfusion scintigraphy** for the detection and localisation of coronary artery disease (angina pectoris and myocardial infarction). - **Assessment of global ventricular function.** First-pass technique for determination of ejection fraction and/or ECG-triggered, gated SPECT for evaluation of left ventricular ejection fraction, volumes and regional wall motion. - **Scintimammography for the detection of suspected breast cancer** when mammography is equivocal, inadequate or indeterminate. - **Localisation of hyperfunctioning parathyroid tissue** in patients with recurrent or persistent disease in both primary and secondary hyperparathyroidism, and in patients with primary hyperparathyroidism scheduled to undergo initial surgery of the parathyroid glands.
**4\. CONTRAINDICATIONS** Hypersensitivity to the active substances to any of the excipients listed in section 11 or to any of the components of the labelled radiopharmaceutical – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. In myocardial scintigraphy investigations under stress conditions, the general contraindications associated with the induction of ergometric or pharmacological stress should be considered.
V09GA01
technetium (99mTc) sestamibi
Manufacturer Information
QT INSTRUMENTS (S) PTE LTD
CIS bio international
