Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Posology and method of administration:** The solution can be administered intravenously (IV) or intramuscularly (IM). **Adults** The recommended single dose is 10 mg, repeated up to three times daily. Prevention of PONV: a single dose of 10 mg is recommended. The maximum recommended daily dose is 30 mg or 0.5 mg/kg body weight. The injectable treatment duration should be as short as possible and transfer to oral or rectal treatment should be made as soon as possible. Treatment durations beyond 12 weeks should be avoided unless the therapeutic benefit is judged to outweigh the risk to the patient. _Elderly_ In elderly patients a dose reduction should be considered, based on renal and hepatic function and overall frailty. _Renal impairment_ In patients with severe renal impairment (creatinine clearance ≤15 mL/min), the daily dose should be reduced by 75%. In patients with moderate to severe renal impairment (creatinine clearance 15–60 mL/min), the dose should be reduced by 50%. _Hepatic impairment_ In patients with severe hepatic impairment, the dose should be reduced by 50%. **Children (aged 1–18 years)** The recommended dose is 0.10 to 0.15 mg/kg body weight, repeated up to three times daily by the intravenous route. The recommended maximum dose in 24 hours is 0.5 mg/kg body weight. Dosing table 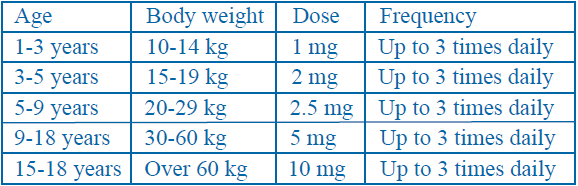 The injectable treatment duration should be as short as possible. The recommended maximum treatment duration is 48 hours for treatment of established post-operative nausea and vomiting (PONV). Metoclopramide is contraindicated in children aged less than 1 year. ( _See “Contraindications”_) Due to the potential risk of severe cardiovascular reactions including cardiac arrest, the solutions for injection are restricted to be used only when appropriate resuscitation equipment is available. ( _See “Undesirable effects, Cardiovascular disorders”_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) **Method of administration** IV doses should be administered as a slow bolus (at least over 3 minutes). A minimum interval of 6 hours between two administrations is to be respected, even in case of vomiting or rejection of dose ( _See “Special warnings”_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**Therapeutic indications:** Adults: - Prevention of nausea and vomiting associated with chemotherapy and radiotherapy with low and minimal emetogenicity - Prevention of post-operative nausea and vomiting - Symptomatic treatment of acute migraine-induced nausea and vomiting - Adjunct treatment of gastroparesis - Adjuvant to surgical and radiological procedures Children (aged 1–18 years): - Treatment of established post-operative nausea and vomiting (PONV) as a second-line option (intravenous use only)
**Contraindications:** This medicinal product is CONTRAINDICATED in the following situations: - children less than 1 year of age, - hypersensitivity to metoclopramide or to one of the other ingredients in the product, - if stimulation of gastrointestinal motility is hazardous to the patient: in the event of gastrointestinal bleeding, mechanical obstruction or gastrointestinal perforation, - in patients having previously presented with tardive dyskinesia induced by neuroleptics or metoclopramide, - in patients with known or suspected pheochromocytoma (apart from as a provocative test); serious hypertensive events have been reported with dopamine antagonists including certain benzamides in this patient category, - in combination with dopamine agonists and selegiline (see _“Interactions with other medicinal products and other forms of interaction”_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), - known history of methaemoglobinaemia with metoclopramide or NADH-cytochrome b5 reductase deficiency - Parkinson’s disease
A03FA01
metoclopramide
Manufacturer Information
YUNG SHIN PHARMACEUTICAL (SINGAPORE) PTE LTD
YUNG SHIN PHARMACEUTICAL IND CO LTD
Active Ingredients
Documents
Package Inserts
Attachment 6 Proposed Clean PI.pdf
Approved: October 8, 2020
