Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**RECOMMENDED DOSAGE** Posology **Dissolution of cholesterol gallstones** The recommended dose in adult patients dependent on the body weight (10 mg/kg/day). The total daily dose should be administered as one single dose, in the evening before going to bed. Ursosan needs to be taken regularly. 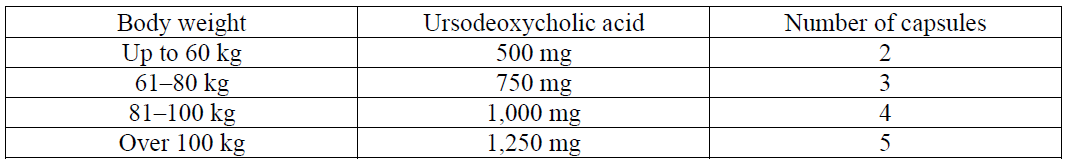 The time required for dissolution of gallstones is generally 6 to 24 months, depending on stone size and composition. If there is no reduction in the size of the gallstones after 12 months, the therapy should not be continued. The success of treatment should be checked sonographically or radiographically every 6 months. At the follow-up examinations, a check should be made to see whether calcification of the stones has occurred in the meantime. Should this be the case, the treatment must be ended. **Treatment of bile reflux gastritis** The recommended dose is 1 capsule of Ursosan 250 mg capsules once daily in the evening before bedtime. For the treatment of bile reflux gastritis Ursosan should normally be taken for 10–14 days. In general the duration of use depends on the course of the disease. The treating physician will decide on the duration of use in the individual case. **Symptomatic treatment of primary biliary cirrhosis (PBC)** The daily dose depends on the body weight and ranges from 3 to 7 capsules (14 ± 2 mg UDCA per kg body weight). For the first 3 months of treatment, Ursosan 250mg capsules should be taken in divided doses throughout the day. When the liver function parameters improve, the daily dose may be taken once daily in the evening. 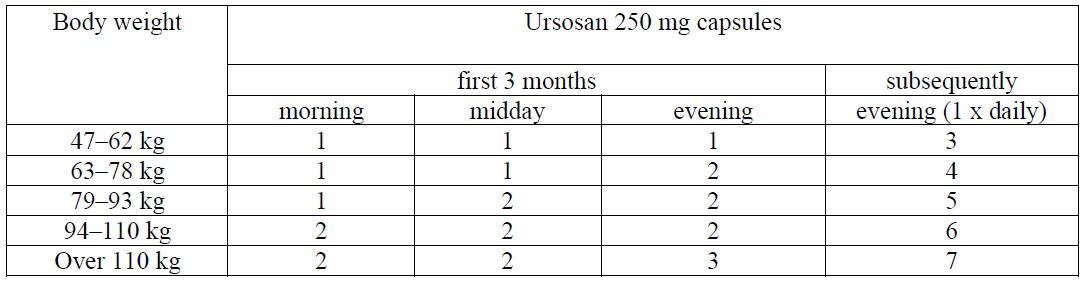 The capsules should be swallowed whole and not chewed, with a sufficient amount of fluids. The capsules must be taken regularly. The use of Ursosan 250 mg capsules in primary biliary cirrhosis may be continued indefinitely. In rare cases, clinical symptoms of the disease may worsen in patients with PBC, for example, the pruritus may worsen. In such a case the Ursosan dose should be reduced to one 250 mg capsule of Ursosan daily and then the dose should be gradually increased again by one capsule weekly until the originally prescribed dose is reached.
ORAL
Medical Information
**INDICATION** - Dissolution of cholesterol gallstones in the gallbladder. The gallstones must not show as shadows on X-ray images and should not exceed 15 mm in diameter. The gallbladder function must not be significantly impaired, despite the gallstones. - Treatment of bile reflux gastritis. - Symptomatic treatment of primary biliary cirrhosis (PBC), in patients without decompensated hepatic cirrhosis.
**CONTRAINDICATIONS** - Hypersensitivity to the active substance or to any of the excipients; - Acute inflammation of the gallbladder or bile ducts; - Occlusion of extrahepatic bile ducts (choledochus duct or cystic duct); - Frequent episodes of biliary colic; - Radio-opaque calcified gallstones; - Impaired contractility of the gallbladder.
A05AA02
ursodeoxycholic acid
Manufacturer Information
PHARMENG TECHNOLOGY PTE. LTD.
PRO.MED.CS Praha a. s.
COOPHARMA s.r.o (Primary and Secondary packager)
SVUS Pharma a.s. (Primary and Secondary packager)
