Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION
**Posology and method of administration** Replacement therapy should be initiated and monitored under a physician experienced in the treatment of immunodeficiency. **Posology** The dose and dosage regimen are dependent on the indication. In replacement therapy the dosage may need to be individualised for each patient depending on the pharmacokinetic and clinical response. The following dosage regimens are given as a guideline. Replacement therapy in primary immunodeficiency syndromes The dosage regimen should achieve a trough level of IgG (measured before the next infusion) of at least 4–6 g/l. Three to six months are required after the initiation of therapy for equilibration to occur. The recommended starting dose is 0.4–0.8 g/kg body weight (BW) followed by at least 0.2 g/kg BW every three weeks. The dose required to achieve a trough level of 6 g/l is of the order of 0.2–0.8 g/kg BW/month. The dosage interval when steady state has been reached varies from 2 to 4 weeks. Trough levels should be measured in order to adjust the dose and dosage interval. Replacement therapy in myeloma or chronic lymphocytic leukaemia (CLL) with severe secondary hypogamma-globulinaemia and recurrent infections; replacement therapy in children with congenital AIDS and recurrent infections The recommended dose is 0.2–0.4 g/kg BW every three to four weeks. Idiopathic Thrombocytopenic Purpura (ITP) For the treatment of an acute episode, 0.8–1 g/kg BW on day one, which may be repeated once within 3 days, or 0.4 g/kg BW daily for two to five days. The treatment can be repeated if a relapse occurs. Guillain Barré Syndrome 0.4 g/kg BW/day for 3 to 7 days. Experience in children is limited. Kawasaki Disease 1.6–2.0 g/kg BW should be administered in divided doses over two to five days or 2.0 g/kg BW as a single dose. Patients should receive concomitant treatment with acetylsalicylic acid. Multifocal Motor Neuropathy (MMN) Starting dose: 2 g/kg given over 2 – 5 days. Maintenance dose: 0.5 – 2.4 g/kg every month based on clinical response. Allogeneic Bone Marrow Transplantation Human normal immunoglobulin treatment can be used as part of the conditioning regimen and after the transplant. For the treatment of infections and prophylaxis of graft versus host disease, dosage is individually tailored. The starting dose is normally 0.5 g/kg BW/week, starting seven days before transplantation and for up to 3 months after transplantation. In case of persistent lack of antibody production, dosage of 0.5 g/kg BW/month is recommended until the antibody level returns to normal. The dosage recommendations are summarised in the following table: 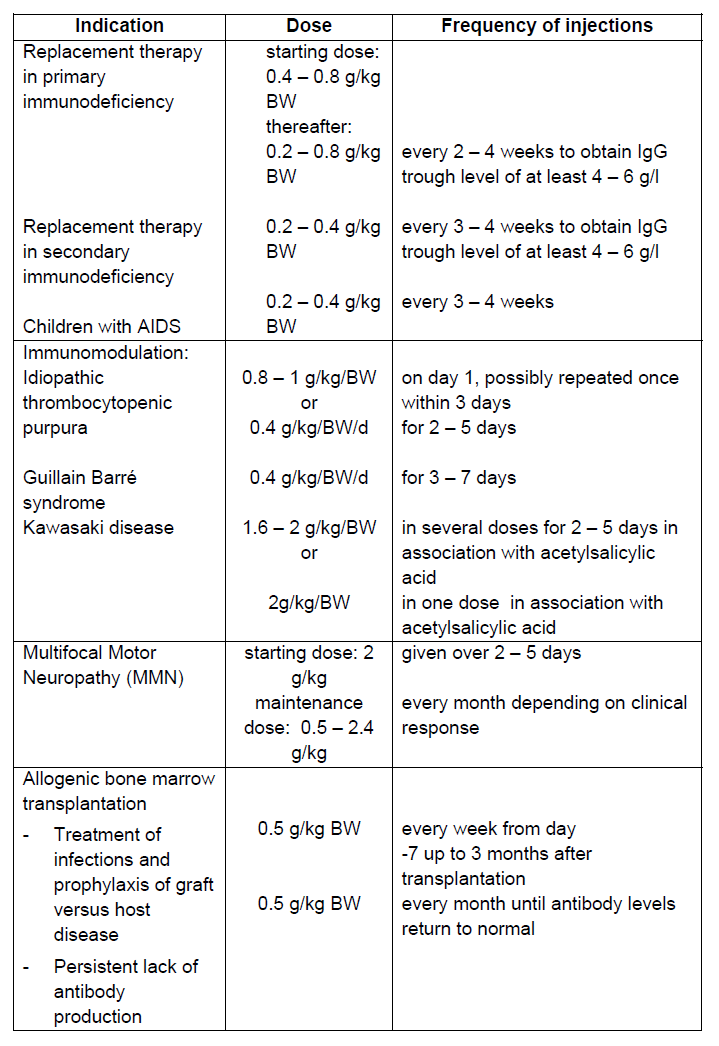 **Method of administration** Human normal immunoglobulin should be infused intravenously at an initial rate of 0.5 ml/kg BW/hr for 30 minutes. If well tolerated, the rate of administration may gradually be increased to a maximum of 6 ml/kg BW/hr. Clinical data obtained from a limited number of patients also indicate that adult PID patients may tolerate an infusion rate of up to 8 ml/kg BW/hr. For further precautions for use see ‘Special warnings and precaution for use’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. In patients at risk for acute renal failure or thromboembolic adverse reactions, KIOVIG should not be infused rapidly. In general, it is recommended that patients beginning treatment with KIOVIG or switching from one intravenous immunoglobulin (IVIg) brand to KIOVIG be started at the lowest rate and then increased to the maximal rate if they have tolerated several infusions at intermediate rates of infusion. KIOVIG should only be administered intravenously. Other routes of administration have not been evaluated. Certain adverse reactions such as headaches and flushing may be related to the rate of infusion. Slowing or stopping the infusion usually allows the symptoms to disappear promptly. The infusion may then be resumed at a rate that does not result in recurrence of the symptoms. (See ‘Adverse Reactions’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) Adverse reactions may occur more frequently in immune deficient patients, who receive human normal immunoglobulin for the first time, when they switch from another IVIg brand, or when there has been a long interval since the previous infusion. (See ‘Adverse Reactions’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) KIOVIG is recommended for infusion at a concentration of 10%. If KIOVIG must be diluted, 5% glucose solution should be used as a diluent. For details on dilution, see ‘Special precautions for disposal and other handling’ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**Therapeutic indications** Replacement therapy in Primary immunodeficiency syndromes such as: - congenital agammaglobulinaemia and hypogamma-globulinaemia - common variable immunodeficiency - severe combined immunodeficiency - Wiskott Aldrich syndrome Myeloma or chronic lymphocytic leukaemia (CLL) with severe secondary hypogammaglobulinaemia and recurrent infections. Children with congenital AIDS and recurrent infections. Immunomodulation - Idiopathic thrombocytopenic purpura (ITP), in children or adults at high risk of bleeding or prior to surgery to correct the platelet count. - Guillain Barré syndrome - Kawasaki disease - Multifocal Motor Neuropathy (MMN) Allogeneic bone marrow transplantation
**Contraindications** KIOVIG is contraindicated in patients with known anaphylactic or severe hypersensitivity responses to Immunoglobulin (Human) or to any of its excipients. KIOVIG is contraindicated in patients who are deficient in IgA and have developed anti-IgA antibodies. Patients with severe IgA deficiency (IgA < 0.05 g/l) may develop anti-IgA antibodies that can result in a severe anaphylactic reaction. Anaphylaxis has been reported with the use of KIOVIG even though it contains low amounts of IgA (average concentration of 37 mcg/ml).
J06BA02
immunoglobulins, normal human, for intravascular adm.
Manufacturer Information
TAKEDA PHARMACEUTICALS (ASIA PACIFIC) PTE. LTD.
Baxalta Belgium Manufacturing S.A.
Active Ingredients
Documents
Package Inserts
Kiovig Solution for Infusion PI.pdf
Approved: February 21, 2017
