Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SUSPENSION
**Posology and method of administration** This medicinal product should always be given by a physician or other qualified health personnel. The usual adult dosage is up to one vial of the product containing 16 microlitre of perfluorobutane (PFB) microbubbles (MB) suspended in 2 ml of the accompanying sterile water for reconstitution to make an 8 microlitre/ml suspension. The product is for intravenous use only and the usual dosage is as per the table below. Before administering Sonazoid, see Special precautions for disposal and other handling for instructions for reconstitution and use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The reconstituted product is for intravenous use. No special preparation of the patient is required. Ultrasound imaging must be performed during injection of Sonazoid as optimal contrast effect is obtained immediately after administration. The intravenous line must be flushed immediately with 5–10 ml sodium chloride 0.9% solution for injection to ensure complete administration of the contrast agent. The recommended clinical dose is 0.12 microlitre PFB microbubbles/kg body weight (is equivalent to 0.015 ml/kg as a suspension). Refer to the table below for weight-based dosages. 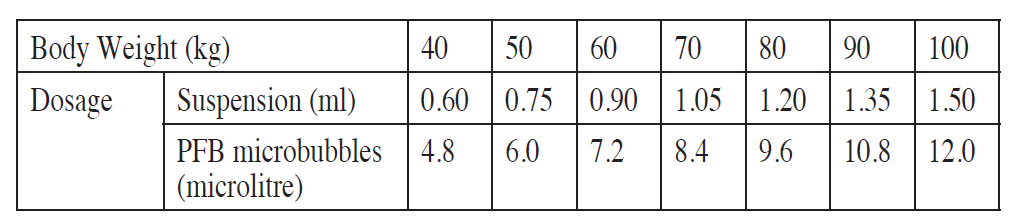 Use in elderly The usual/proposed dose for adults can be used. Paediatric use The safety of this product has not been established in the paediatric population (no data are available).
INTRAVENOUS
Medical Information
**Therapeutic indications** This medicinal product is for diagnostic use only. Sonazoid is an ultrasound contrast agent for use in vascular phase and Kupffer phase for ultrasonic imaging of focal hepatic lesions.
**Contraindications** Hypersensitivity to the active substance or to any of the excipients.
V08DA06
perflubutane, phospholipid microspheres
Manufacturer Information
GE HEALTHCARE PTE. LTD.
GE Healthcare AS (Oslo Site)
B. Braun Melsungen AG (Solvent)
Active Ingredients
Documents
Package Inserts
Sonazoid PI.pdf
Approved: October 26, 2021
