Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SUSPENSION
**Dosage and Administration** It is recommended to take oral MOTILIUM® 15–30 minutes before meals. If taken after meals, absorption of the drug is somewhat delayed. **Adults and adolescents ≥ 12 years of age and weighing ≥ 35 kg** The dose of MOTILIUM® should be the lowest effective dose for the individual situation (typically 30mg/day) and can be increased if necessary to a maximum daily oral dose of 40mg. Usually, the maximum treatment duration should not exceed one week for the treatment of acute nausea and vomiting. If nausea and vomiting persists for longer than one week, patients should consult their physician. For other indications, the initial duration of treatment is up to four weeks. If treatment exceeds four weeks, patients should be re-evaluated and the need for continued treatment reassessed. 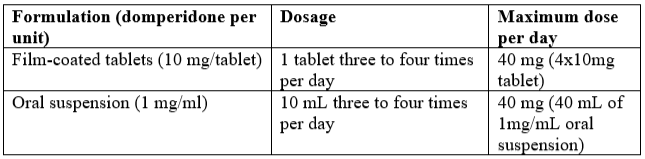 Doses above 30mg/day should be used with caution due to potential risk of arrhythmias associated with the higher doses. **Infants and children < 12 years of age and weighing < 35 kg** The efficacy of MOTILIUM® has not been established in infants and children < 12 years of age and weighing < 35 kg (see _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Use in renal insufficiency** Since the elimination half-life of domperidone is prolonged in severe renal impairment (serum creatinine > 6mg/100mL, i.e. > 0.6mmol/L, the dosing frequency of MOTILIUM® should be reduced to once or twice daily, depending on the severity of the impairment, and the dose may need to be reduced. Patients with severe renal impairment should be reviewed regularly (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** MOTILIUM® is contraindicated for patients with moderate (Child-Pugh 7 to 9) or severe (Child-Pugh >9) hepatic impairment (see _Contraindications_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dose adjustment is not required for patients with mild (Child-Pugh 5 to 6) hepatic impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**Indications** 1. The dyspeptic symptom complex that is often associated with delayed gastric emptying, gastro-esophageal reflux and esophagitis: - epigastric sense of fullness, early satiety, feeling of abdominal distension, upper abdominal pain; - bloating, eructation, flatulence; - nausea and vomiting; - heartburn with or without regurgitations of gastric contents in the mouth. 2. Nausea and vomiting of functional, organic, infectious or dietary origin. 3. Nausea and vomiting induced by: - radiotherapy or drug therapy - dopamine agonists (such as L-dopa and bromocriptine) used in the treatment of Parkinson’s disease.
**Contraindications** MOTILIUM® is contraindicated in the following situations: - Known hypersensitivity to domperidone or any of the excipients - Prolactin-releasing pituitary tumour (prolactinoma) - Co-administration with QT-prolonging drugs (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - Co-administration with potent CYP3A4 inhibitors (see _Interactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - In patients who have known existing prolongation of cardiac conduction of intervals, particularly QTc, patients with significant electrolyte disturbances or underlying cardiac diseases such as congestive heart failure (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - Whenever stimulation of gastric motility might be dangerous, e.g. in the presence of gastro-intestinal haemorrhage, mechanical obstruction or perforation - In patients with moderate or severe hepatic impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_)
A03FA03
domperidone
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
JANSSEN PHARMACEUTICA NV
Active Ingredients
Documents
Package Inserts
Motilium Suspension PI.pdf
Approved: December 6, 2022
