Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
PATCH
**Dosage and Administration** DUROGESIC® doses should be individualized based upon the status of the patient and should be assessed at regular intervals after application. The lowest effective dose should be used. The patches are designed to deliver approximately 12, 25, 50, 75, and 100 mcg/hour fentanyl to the systemic circulation, which represent about 0.3, 0.6, 1.2, 1.8, and 2.4 mg per day (see _Dosage Forms and Strengths_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), respectively. **Initial Dosage Selection** The appropriate initiating dose of DUROGESIC® should be based on the patient’s current opioid use. It is recommended that DUROGESIC® be used in patients who have demonstrated opioid tolerance. Other factors to be considered are the current general condition and medical status of the patient, including body size, age, and extent of debilitation as well as degree of opioid tolerance. **Dosage – Adults** **_Opioid-tolerant patients_** To convert opioid-tolerant patients from oral or parenteral opioids to DUROGESIC®, refer to _Equianalgesic potency conversion_ below. The dosage may subsequently be titrated upwards or downwards, if required, in increments of either 12 or 25 mcg/hour depending on response and supplementary analgesic requirements. _**Equianalgesic Potency Conversion**_ 1. Calculate the previous 24-hour analgesic requirement. 2. Convert this amount to the equianalgesic oral morphine dose using Table 1. All Intramuscular (IM) and oral doses in this chart are considered equivalent to 10 mg of IM morphine in analgesic effect. 3. To derive the DUROGESIC® dosage corresponding to the calculated 24-hour, equianalgesic morphine dosage, use the dosage-conversion Table 2 as follows: Table 2 is for adult patients who have a need for rotation of, or conversion from, another opioid regimen (conversion ratio of oral morphine to transdermal fentanyl approximately equal to 150:1). 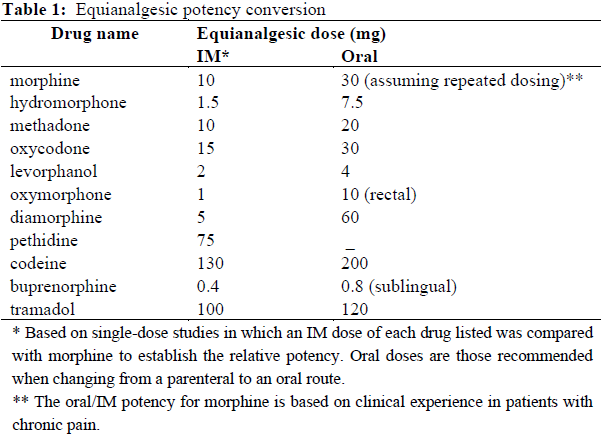 Reference: Adapted from Foley KM. The treatment of cancer pain. N Engl J Med 1985;313(2):84–95 and McPherson ML. Introduction to opioid conversion calculations. In: Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing. Bethesda, MD: American Society of Health-System Pharmacists; 2010:1–15. 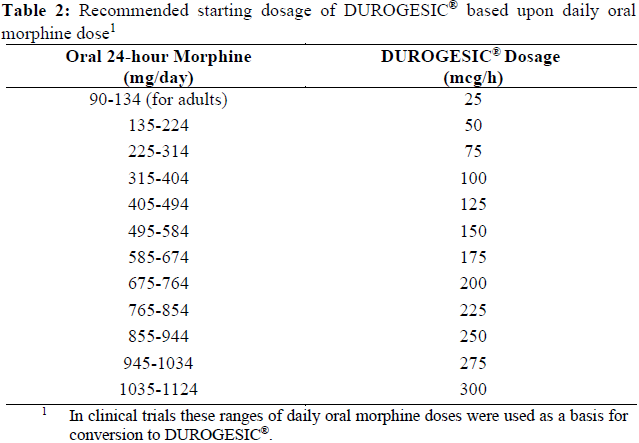 Initial evaluation of the maximum analgesic effect of DUROGESIC® cannot be made before the patch is worn for 24 hours. This delay is due to the gradual increase in serum fentanyl concentration in the 24 hours following initial patch application. Previous analgesic therapy should therefore be gradually phased out after the initial dose application until analgesic efficacy with DUROGESIC® is attained. _**Dose Titration and Maintenance Therapy**_ **General** - Replace the patch every 72 hours. - If the patch needs to be replaced (e.g., the patch falls off) before 72 hours, apply a patch of the same strength to a different skin site. This may result in increased serum concentrations (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) therefore monitor the patient closely. - More than one DUROGESIC® patch may be used for doses greater than 100 mcg/hour. - At any point during treatment, a patient may require periodic supplemental doses of a short acting analgesic for “breakthrough” pain. Some patients may require additional or alternative methods of opioid administration when the DUROGESIC® dose exceeds 300 mcg/hour. **First Patch Application** If analgesia is insufficient, during the first application: - Replace the DUROGESIC® patch with a patch of the same dose after 48 hours OR - Increase the dose when a new patch is applied after 72 hours (see _Dose Titration_ below). **Dose Titration** - Titrate the dose individually based on average daily use of supplemental analgesics, until a balance between analgesic efficacy and tolerability is attained. - A 12 mcg/hour strength is available for dose titration. Dosage titration is normally in 12 mcg/h or 25 mcg/hour increments, although the supplementary analgesic requirements (oral morphine 45/90 mg/day ≈ DUROGESIC® 12/25 mcg/hour) and pain status of the patient should be taken into account. - After an increase in dose, it may take up to 6 days for the patient to reach equilibrium on the new dose level. Therefore after a dose increase, patients should wear the higher dose patch through two 72-hour applications before increasing the dose further. **Maintenance Therapy** - The principles described under General above are applicable during maintenance therapy. **Dosage – Pediatrics** DUROGESIC® should be administered only to opioid-tolerant pediatric patients (ages 2 to 16 years) who are already receiving at least 45mg oral morphine equivalents per day. To convert pediatric patients from oral or parenteral opioids to DUROGESIC®, refer to _Equianalgesic potency conversion_ (Table 1) and _Recommended DUROGESIC® dosage based upon daily oral morphine dose_ (Table 3).  **Discontinuation of DUROGESIC®** If discontinuation of DUROGESIC® is necessary, replacement with other opioids should be gradual, starting at a low dose and increasing slowly. This is because while fentanyl concentrations fall gradually after DUROGESIC® is removed, it takes 20 hours or more for the fentanyl serum concentrations to decrease 50%. In general, the discontinuation of opioid analgesia should be gradual in order to prevent withdrawal symptoms. There have been reports that rapid discontinuation of opioid analgesics in patients who are physically dependent on opioids has resulted in serious withdrawal symptoms and uncontrolled pain. Opioid withdrawal symptoms (see _Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) are possible in some patients after conversion or dose adjustment. Table 1 and Table 2 should not be used to convert from DUROGESIC® to other therapies to avoid overestimating the new analgesic dose and potentially causing overdose.
TRANSDERMAL
Medical Information
**Indications** DUROGESIC® is indicated in the management of chronic pain and intractable pain requiring opioid analgesia that requires continuous opioid administration for an extended period of time in opioid-tolerant children age 2 years and older and in adults.
**Contraindications** DUROGESIC® is contraindicated in patients with known hypersensitivity to fentanyl or to the adhesives present in the patch. Because serious or life-threatening hypoventilation could occur and because there is no opportunity for dose titration during short-term use, DUROGESIC® is contraindicated: - in the management of acute pain or in patients who require opioid analgesia for a short period of time - in the management of post-operative pain, including use after out-patient or day surgeries (eg tonsillectomies) - in the management of mild pain - in the management of intermittent pain (eg use on an as needed basis (prn) - in situations of significant respiratory depression, especially in unmonitored settings where there is a lack of resuscitative equipment - in patients who have acute or severe bronchial asthma DUROGESIC® is contraindicated in patients who have or are suspected of having paralytic ileus.
N02AB03
fentanyl
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Alza Ireland Ltd
Janssen Pharmaceutica NV
Active Ingredients
Documents
Package Inserts
DUROGESIC PI.pdf
Approved: December 6, 2022
